FULL PRESCRIBING INFORMATION
WARNING: RISK OF THYROID C-CELL TUMORS
- In rats, tirzepatide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether ZEPBOUND causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined [see Warnings and Precautions (5.1) and Nonclinical Toxicology (13.1)].
- ZEPBOUND is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) [see Contraindications (4)]. Counsel patients regarding the potential risk for MTC with the use of ZEPBOUND and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with ZEPBOUND [see Contraindications (4) and Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
ZEPBOUND® is indicated in combination with a reduced-calorie diet and increased physical activity to reduce excess body weight and maintain weight reduction long term in adults with obesity or adults with overweight in the presence of at least one weight-related comorbid condition.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
- The recommended starting dosage of ZEPBOUND is 2.5 mg injected subcutaneously once weekly. Follow the dosage escalation below to reduce the risk of gastrointestinal adverse reactions [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)]. The 2.5 mg dosage is for treatment initiation and is not approved as a maintenance dosage.
- After 4 weeks, increase the dosage to 5 mg injected subcutaneously once weekly. The dosage may be increased in 2.5 mg increments, after at least 4 weeks on the current dose.
- The recommended maintenance dosages of ZEPBOUND in adults are 5 mg, 10 mg, or 15 mg, injected subcutaneously once weekly.
- Consider treatment response and tolerability when selecting the maintenance dosage [see Adverse Reactions (6.1) and Clinical Studies (14.1)]. If patients do not tolerate a maintenance dosage, consider a lower maintenance dosage.
- The maximum dosage of ZEPBOUND is 15 mg injected subcutaneously once weekly.
2.2 Recommendations Regarding Missed Dose
- If a dose is missed, instruct patients to administer ZEPBOUND as soon as possible within 4 days (96 hours) after the missed dose. If more than 4 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once weekly dosing schedule.
- The day of weekly administration can be changed, if necessary, as long as the time between the two doses is at least 3 days (72 hours).
2.3 Important Administration Instructions
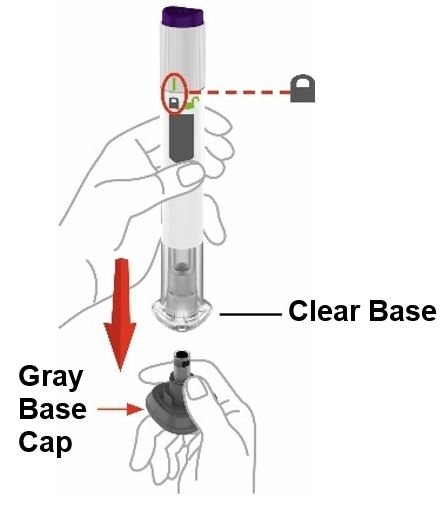
- Prior to initiation of ZEPBOUND, train patients and caregivers on proper injection technique. Refer to the accompanying Instructions for Use for complete administration instructions with illustrations.
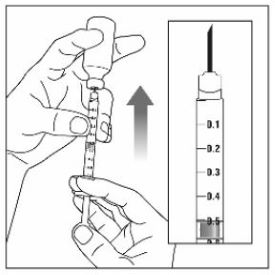
- Instruct patients using the single-dose vial to use a syringe appropriate for dose administration (e.g., a 1 mL syringe capable of measuring a 0.5 mL dose).
- Inspect ZEPBOUND visually before use. It should appear clear and colorless to slightly yellow. Do not use ZEPBOUND if particulate matter or discoloration is seen.
- Administer ZEPBOUND in combination with a reduced-calorie diet and increased physical activity.
- Administer ZEPBOUND once weekly at any time of day, with or without meals.
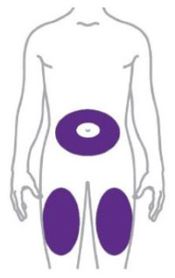
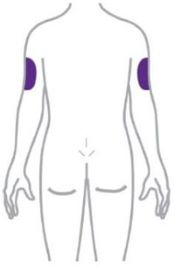
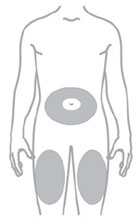
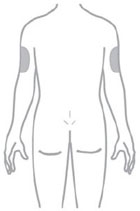
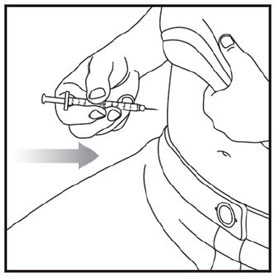
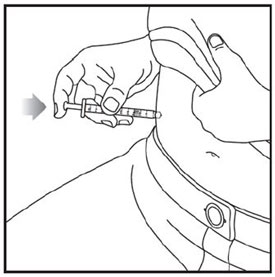
- Inject ZEPBOUND subcutaneously in the abdomen, thigh, or upper arm.
- Rotate injection sites with each dose.
3 DOSAGE FORMS AND STRENGTHS
Injection: Clear, colorless to slightly yellow solution in pre-filled single-dose pens or single-dose vials, each available in the following strengths:
- 2.5 mg/0.5 mL
- 5 mg/0.5 mL
- 7.5 mg/0.5 mL
- 10 mg/0.5 mL
- 12.5 mg/0.5 mL
- 15 mg/0.5 mL
4 CONTRAINDICATIONS
ZEPBOUND is contraindicated in patients with:
- A personal or family history of MTC or in patients with MEN 2 [see Warnings and Precautions (5.1)].
- Known serious hypersensitivity to tirzepatide or any of the excipients in ZEPBOUND. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with tirzepatide [see Warnings and Precautions (5.6) and Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Thyroid C-Cell Tumors
In rats, tirzepatide caused a dose-dependent and treatment-duration-dependent increase in the incidence of thyroid C-cell tumors (adenomas and carcinomas) in a 2-year study at clinically relevant plasma exposures [see Nonclinical Toxicology (13.1)]. It is unknown whether ZEPBOUND causes thyroid C-cell tumors, including MTC, in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined.
ZEPBOUND is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2. Counsel patients regarding the potential risk for MTC with the use of ZEPBOUND and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness).
Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with ZEPBOUND. Such monitoring may increase the risk of unnecessary procedures, due to the low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin values may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
5.2 Severe Gastrointestinal Adverse Reactions
Use of ZEPBOUND has been associated with gastrointestinal adverse reactions, sometimes severe [see Adverse Reactions (6.1)]. In a pool of two ZEPBOUND clinical trials (Studies 1 and 2), severe gastrointestinal adverse reactions were reported more frequently among patients receiving ZEPBOUND (5 mg 1.7%, 10 mg 2.5%, 15 mg 3.1%) than placebo (1%).
ZEPBOUND has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients.
5.3 Acute Kidney Injury
Use of ZEPBOUND has been associated with acute kidney injury, which can result from dehydration due to gastrointestinal adverse reactions to ZEPBOUND, including nausea, vomiting, and diarrhea [see Adverse Reactions (6.1)].
In patients treated with GLP-1 receptor agonists, there have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, which may sometimes require hemodialysis. Some of these events have been reported in patients without known underlying renal disease. A majority of the reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration. Monitor renal function in patients reporting adverse reactions to ZEPBOUND that could lead to volume depletion.
5.4 Acute Gallbladder Disease
Treatment with ZEPBOUND and GLP-1 receptor agonists is associated with an increased occurrence of acute gallbladder disease.
In a pool of two ZEPBOUND clinical trials (Studies 1 and 2), cholelithiasis was reported in 1.1% of ZEPBOUND-treated patients and 1% of placebo-treated patients, cholecystitis was reported in 0.7% of ZEPBOUND-treated patients and 0.2% of placebo-treated patients, and cholecystectomy was reported in 0.2% of ZEPBOUND-treated patients and no placebo-treated patients. Acute gallbladder events were associated with weight reduction. If cholecystitis is suspected, gallbladder diagnostic studies and appropriate clinical follow-up are indicated.
5.5 Acute Pancreatitis
Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists or tirzepatide.
In clinical trials of tirzepatide for a different indication, 14 events of acute pancreatitis were confirmed by adjudication in 13 tirzepatide-treated patients (0.23 patients per 100 years of exposure) versus 3 events in 3 comparator-treated patients (0.11 patients per 100 years of exposure). In a pool of two ZEPBOUND clinical trials (Studies 1 and 2), 0.2% of ZEPBOUND-treated patients had acute pancreatitis confirmed by adjudication (0.14 patients per 100 years of exposure) versus 0.2% of placebo-treated patients (0.15 patients per 100 years of exposure). ZEPBOUND has not been studied in patients with a prior history of pancreatitis. It is unknown if patients with a history of pancreatitis are at higher risk for development of pancreatitis on ZEPBOUND.
After initiation of ZEPBOUND, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting). If pancreatitis is suspected, discontinue ZEPBOUND and initiate appropriate management. If the diagnosis of pancreatitis is confirmed, ZEPBOUND should not be restarted.
5.6 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) in patients treated with tirzepatide. In a pool of two ZEPBOUND clinical trials (Studies 1 and 2), 0.1% of ZEPBOUND-treated patients had severe hypersensitivity reactions compared to no placebo-treated patients. If hypersensitivity reactions occur, advise patients to promptly seek medical attention and discontinue use of ZEPBOUND. Do not use in patients with a previous serious hypersensitivity reaction to tirzepatide or any of the excipients in ZEPBOUND [see Contraindications (4) and Adverse Reactions (6.2)].
Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with GLP-1 receptor agonists. Use caution in patients with a history of angioedema or anaphylaxis with a GLP-1 receptor agonist because it is unknown whether such patients will be predisposed to these reactions with ZEPBOUND.
5.7 Hypoglycemia
ZEPBOUND lowers blood glucose and can cause hypoglycemia.
In a trial of patients with type 2 diabetes mellitus and BMI ≥27 kg/m2 (Study 2), hypoglycemia (plasma glucose <54 mg/dL) was reported in 4.2% of ZEPBOUND-treated patients versus 1.3% of placebo-treated patients. In this trial, patients taking ZEPBOUND in combination with an insulin secretagogue (e.g., sulfonylurea) had increased risk of hypoglycemia (10.3%) compared to ZEPBOUND-treated patients not taking a sulfonylurea (2.1%). There is also increased risk of hypoglycemia in patients treated with tirzepatide in combination with insulin [see Drug Interactions (7.1)].
Hypoglycemia has also been associated with ZEPBOUND and GLP-1 receptor agonists in adults without type 2 diabetes mellitus [see Adverse Reactions (6.1)].
Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia. In patients with diabetes mellitus, monitor blood glucose prior to starting ZEPBOUND and during ZEPBOUND treatment. The risk of hypoglycemia may be lowered by a reduction in the dose of insulin or sulfonylurea (or other concomitantly administered insulin secretagogue).
5.8 Diabetic Retinopathy Complications in Patients with Type 2 Diabetes Mellitus
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Tirzepatide has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
5.9 Suicidal Behavior and Ideation
Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients treated with ZEPBOUND for the emergence or worsening of depression, suicidal thoughts or behaviors, and/or any unusual changes in mood or behavior. Discontinue ZEPBOUND in patients who experience suicidal thoughts or behaviors. Avoid ZEPBOUND in patients with a history of suicidal attempts or active suicidal ideation.
5.10 Pulmonary Aspiration During General Anesthesia or Deep Sedation
ZEPBOUND delays gastric emptying [see Clinical Pharmacology (12.2)]. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations.
Available data are insufficient to inform recommendations to mitigate the risk of pulmonary aspiration during general anesthesia or deep sedation in patients taking ZEPBOUND, including whether modifying preoperative fasting recommendations or temporarily discontinuing ZEPBOUND could reduce the incidence of retained gastric contents. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking ZEPBOUND.
6 ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Risk of Thyroid C-cell Tumors [see Warnings and Precautions (5.1)]
- Severe Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.2)]
- Acute Kidney Injury [see Warnings and Precautions (5.3)]
- Acute Gallbladder Disease [see Warnings and Precautions (5.4)]
- Acute Pancreatitis [see Warnings and Precautions (5.5)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.6)]
- Hypoglycemia [see Warnings and Precautions (5.7)]
- Diabetic Retinopathy Complications in Patients with Type 2 Diabetes Mellitus [see Warnings and Precautions (5.8)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.9)]
- Pulmonary Aspiration During General Anesthesia or Deep Sedation [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Weight Reduction Trials in Adults with Obesity or Overweight, with or without Type 2 Diabetes (Study 1 and Study 2)
ZEPBOUND was evaluated for safety in a pool of two randomized, double-blind, placebo-controlled trials that included 2,519 adult patients with obesity or overweight treated with ZEPBOUND for up to 72 weeks and a 4-week off drug follow-up period (Study 1 and Study 2) [see Clinical Studies (14.1)]. The mean age of patients was 47 years and 37% were male. The population was 72% White, 12% Asian, 8% Black or African American, and 7% American Indian or Alaska Native; 51% identified as Hispanic or Latino ethnicity. Baseline characteristics included an average BMI of 37.4 kg/m2, 29% with a BMI ≥40 kg/m2, 41% with hypertension, 37% with dyslipidemia, 25% with type 2 diabetes mellitus, 7% with obstructive sleep apnea, and 4% with cardiovascular disease.
Across both trials, 4.8%, 6.3%, and 6.7% of patients treated with 5 mg, 10 mg, and 15 mg of ZEPBOUND, respectively, permanently discontinued treatment as a result of adverse reactions compared to 3.4% of patients treated with placebo. The majority of patients who discontinued ZEPBOUND due to adverse reactions did so during the first few months of treatment due to gastrointestinal adverse reactions.
Common Adverse Reactions
Table 1 shows common adverse reactions associated with the use of ZEPBOUND in the pool of two placebo-controlled trials for weight reduction (Study 1 and Study 2). These adverse reactions occurred more commonly with ZEPBOUND than with placebo and occurred in at least 2% of patients treated with ZEPBOUND.
|
a Includes diarrhea, frequent bowel movements. |
||||
|
b Includes constipation, feces hard. |
||||
|
c Includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, abdominal tenderness. |
||||
|
d Includes multiple related adverse event terms, such as injection site bruising, injection site erythema, injection site pruritus, injection site pain, injection site rash, injection site reaction. |
||||
|
e Includes asthenia, fatigue, lethargy, malaise. |
||||
|
f Includes blood pressure decreased, hypotension, orthostatic hypotension. |
||||
Adverse Reaction |
Placebo (N=958) % |
ZEPBOUND 5 mg (N=630) % |
ZEPBOUND 10 mg (N=948) % |
ZEPBOUND 15 mg (N=941) % |
| Nausea | 8 | 25 | 29 | 28 |
| Diarrheaa | 8 | 19 | 21 | 23 |
| Vomiting | 2 | 8 | 11 | 13 |
| Constipationb | 5 | 17 | 14 | 11 |
| Abdominal Painc | 5 | 9 | 9 | 10 |
| Dyspepsia | 4 | 9 | 9 | 10 |
| Injection Site Reactionsd | 2 | 6 | 8 | 8 |
| Fatiguee | 3 | 5 | 6 | 7 |
| Hypersensitivity Reactions | 3 | 5 | 5 | 5 |
| Eructation | 1 | 4 | 5 | 5 |
| Hair Loss | 1 | 5 | 4 | 5 |
| Gastroesophageal Reflux Disease | 2 | 4 | 4 | 5 |
| Flatulence | 2 | 3 | 3 | 4 |
| Abdominal Distension | 2 | 3 | 3 | 4 |
| Dizziness | 2 | 4 | 5 | 4 |
| Hypotensionf | 0 | 1 | 1 | 2 |
In a clinical trial that included an intensive lifestyle intervention lead-in period (Study 3), 287 patients were treated with ZEPBOUND for up to 72 weeks. In a randomized withdrawal trial (Study 4), 783 patients were treated with ZEPBOUND for up to 36 weeks, and 335 of these patients were treated for up to 88 weeks [see Clinical Studies (14.1)]. In Study 3, 10% of ZEPBOUND-treated patients and 2% of placebo-treated patients discontinued drug due to adverse reactions. In Study 4, 7% of patients discontinued ZEPBOUND treatment before randomized withdrawal at Week 36 due to adverse reactions. In Study 3 and Study 4, adverse reactions were similar to those reported in the two pooled ZEPBOUND clinical trials (Study 1 and Study 2).
Gastrointestinal Adverse Reactions
In a pool of Study 1 and 2, gastrointestinal adverse reactions occurred more frequently among patients receiving ZEPBOUND (5 mg 56%, 10 mg 56%, 15 mg 56%) than placebo (30%). More patients receiving ZEPBOUND 5 mg (1.9%), ZEPBOUND 10 mg (3.3%), and ZEPBOUND 15 mg (4.3%) discontinued treatment due to gastrointestinal adverse reactions than patients receiving placebo (0.5%). The majority of nausea, vomiting, and/or diarrhea events occurred during dose escalation and decreased over time.
Hypotension
In a pool of Study 1 and 2, hypotension occurred more frequently among patients taking ZEPBOUND (1.6%) than patients taking placebo (0.1%). Hypotension was more frequently seen in ZEPBOUND-treated patients on concomitant antihypertensive therapy (2.2%) compared to ZEPBOUND-treated patients not on antihypertensive therapy (1.2%). Hypotension also occurred in association with gastrointestinal adverse events and dehydration.
Hypersensitivity Reactions
In a pool of Study 1 and 2, immediate hypersensitivity reactions (within one day after drug administration) occurred in 2.1% of ZEPBOUND-treated patients compared to 0.4% of placebo-treated patients, while non-immediate hypersensitivity reactions occurred in 3.5% of ZEPBOUND-treated patients compared to 2.7% of placebo-treated patients. Among ZEPBOUND-treated patients, hypersensitivity reactions were more frequent in those with anti-tirzepatide antibodies (6.2%) compared to those who did not develop anti-tirzepatide antibodies (3%) [see Clinical Pharmacology (12.6)]. The majority of the hypersensitivity reactions in trials were skin reactions (e.g., rash, itching).
Injection Site Reactions
In ZEPBOUND-treated patients in a pool of Study 1 and 2, injection site reactions were more frequent in those with anti-tirzepatide antibodies (11.3%) compared to those who did not develop anti-tirzepatide antibodies (1%) [see Clinical Pharmacology (12.6)].
Hair Loss
Hair loss adverse reactions in ZEPBOUND-treated patients were associated with weight reduction. In a pool of Study 1 and 2, hair loss was reported more frequently in female than male patients in the ZEPBOUND (7.1% female versus 0.5% male) and placebo (1.3% female versus 0% male) treatment groups. No ZEPBOUND-treated patients and one placebo-treated patient discontinued study treatment due to hair loss.
Other Adverse Reactions
Acute Kidney Injury
In a pool of Study 1 and 2, acute kidney injury was reported in 0.5% of ZEPBOUND-treated patients compared to 0.2% of placebo-treated patients.
Acute Gallbladder Disease
In a pool of Study 1 and 2, cholelithiasis was reported in 1.1% of ZEPBOUND-treated patients and 1% of placebo-treated patients, cholecystitis was reported in 0.7% of ZEPBOUND-treated patients and 0.2% of placebo-treated patients, and cholecystectomy was reported in 0.2% of ZEPBOUND-treated patients and no placebo-treated patients.
Hypoglycemia
In Study 2, a trial of patients with type 2 diabetes mellitus and BMI ≥27 kg/m2, hypoglycemia (plasma glucose <54 mg/dL) was reported in 4.2% of ZEPBOUND-treated patients versus 1.3% of placebo-treated patients.
In Study 1, a trial of ZEPBOUND in adults with obesity/overweight without type 2 diabetes mellitus, there was no systematic capturing of hypoglycemia, but plasma glucose <54 mg/dL was reported in 0.3% of ZEPBOUND-treated patients versus no placebo-treated patients.
Heart Rate Increase
In a pool of Study 1 and 2, treatment with ZEPBOUND resulted in a mean increase in heart rate of 1 to 3 beats per minute compared to no increase in placebo-treated patients.
Dysesthesia
In a pool of Study 1 and 2, dysesthesia occurred more frequently among patients receiving ZEPBOUND (5 mg 0.2%, 10 mg 0.2%, 15 mg 0.4%) than placebo (0.1%).
Laboratory Abnormalities
Amylase and Lipase Increase
In a pool of Study 1 and 2, treatment with ZEPBOUND resulted in mean increases from baseline in serum pancreatic amylase concentrations of 20% to 25% and serum lipase concentrations of 28% to 35%, compared to mean increases from baseline in pancreatic amylase of 2.1% and serum lipase of 5.8% in placebo-treated patients. The clinical significance of elevations in amylase or lipase with ZEPBOUND is unknown in the absence of other signs and symptoms of pancreatitis.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of tirzepatide, the active ingredient in ZEPBOUND. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure.
Hypersensitivity: anaphylaxis, angioedema
Gastrointestinal: ileus
Pulmonary: Pulmonary aspiration has occurred in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation.
7 DRUG INTERACTIONS
7.1 Concomitant Use with Insulin or an Insulin Secretagogue (e.g., Sulfonylurea)
ZEPBOUND lowers blood glucose. When initiating ZEPBOUND, consider reducing the dose of concomitantly administered insulin or insulin secretagogues (e.g., sulfonylureas) to reduce the risk of hypoglycemia [see Warnings and Precautions (5.7)].
7.2 Oral Medications
ZEPBOUND delays gastric emptying and thereby has the potential to impact the absorption of concomitantly administered oral medications. Caution should be exercised when oral medications are concomitantly administered with ZEPBOUND.
Monitor patients on oral medications dependent on threshold concentrations for efficacy and those with a narrow therapeutic index (e.g., warfarin) when concomitantly administered with ZEPBOUND.
Advise patients using oral hormonal contraceptives to switch to a non-oral contraceptive method, or add a barrier method of contraception, for 4 weeks after initiation with ZEPBOUND and for 4 weeks after each dose escalation. Hormonal contraceptives that are not administered orally should not be affected [see Use in Specific Populations (8.3) and Clinical Pharmacology (12.2, 12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There will be a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ZEPBOUND (tirzepatide) during pregnancy. Pregnant patients exposed to ZEPBOUND and healthcare providers are encouraged to contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979).
Risk Summary
Weight loss offers no benefit to a pregnant patient and may cause fetal harm. Advise pregnant patients that weight loss is not recommended during pregnancy and to discontinue ZEPBOUND when a pregnancy is recognized (see Clinical Considerations). Available data with tirzepatide in pregnant patients are insufficient to evaluate for a drug-related risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Based on animal reproduction studies, there may be risks to the fetus from exposure to tirzepatide during pregnancy.
In pregnant rats administered tirzepatide during organogenesis, fetal growth reductions and fetal abnormalities occurred at clinical exposure in maternal rats based on AUC. In rabbits administered tirzepatide during organogenesis, fetal growth reductions were observed at clinically relevant exposures based on AUC. These adverse embryo/fetal effects in animals coincided with pharmacological effects on maternal weight and food consumption (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is increased when compared to the general population. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In pregnant rats given twice weekly subcutaneous doses of 0.02, 0.1, and 0.5 mg/kg tirzepatide [0.03-, 0.07-, and 0.5-fold the maximum recommended human dose (MRHD) of 15 mg once weekly based on AUC] during organogenesis, increased incidences of external, visceral, and skeletal malformations, increased incidences of visceral and skeletal developmental variations, and decreased fetal weights coincided with pharmacologically-mediated reductions in maternal body weights and food consumption at 0.5 mg/kg. In pregnant rabbits given once weekly subcutaneous doses of 0.01, 0.03, or 0.1 mg/kg tirzepatide (0.01-, 0.06-, and 0.2-fold the MRHD) during organogenesis, pharmacologically mediated effects on the gastrointestinal system resulting in maternal mortality or abortion in a few rabbits occurred at all dose levels. Reduced fetal weights associated with decreased maternal food consumption and body weights were observed at 0.1 mg/kg. In a pre- and post-natal study in rats administered subcutaneous doses of 0.02, 0.10, or 0.25 mg/kg tirzepatide twice weekly from implantation through lactation, F1 pups from F0 maternal rats given 0.25 mg/kg tirzepatide had statistically significant lower mean body weight when compared to controls from post-natal day 7 through post-natal day 126 for males and post-natal day 56 for females.
8.2 Lactation
Risk Summary
There are no data on the presence of tirzepatide or its metabolites in animal or human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ZEPBOUND and any potential adverse effects on the breastfed infant from ZEPBOUND or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Use of ZEPBOUND may reduce the efficacy of oral hormonal contraceptives due to delayed gastric emptying. This delay is largest after the first dose and diminishes over time. Advise patients using oral hormonal contraceptives to switch to a non-oral contraceptive method, or add a barrier method of contraception, for 4 weeks after initiation with ZEPBOUND and for 4 weeks after each dose escalation [see Drug Interactions (7.2) and Clinical Pharmacology (12.2, 12.3)].
8.4 Pediatric Use
The safety and effectiveness of ZEPBOUND have not been established in pediatric patients.
8.5 Geriatric Use
In a pool of two fixed dose ZEPBOUND clinical trials (Study 1 and Study 2), 226 (9%) ZEPBOUND-treated patients were 65 years of age or older, and 13 (0.5%) ZEPBOUND-treated patients were 75 years of age or older at baseline.
No overall differences in safety or effectiveness of ZEPBOUND have been observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
No dosage adjustment of ZEPBOUND is recommended for patients with renal impairment. In subjects with renal impairment including end-stage renal disease (ESRD), no change in tirzepatide pharmacokinetics (PK) was observed [see Clinical Pharmacology (12.3)]. Monitor renal function in patients reporting adverse reactions to ZEPBOUND that could lead to volume depletion [see Warnings and Precautions (5.3)].
8.7 Hepatic Impairment
No dosage adjustment of ZEPBOUND is recommended for patients with hepatic impairment. In a clinical pharmacology study in subjects with varying degrees of hepatic impairment, no change in tirzepatide PK was observed [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In the event of an overdosage, contact the Poison Help Line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations. Appropriate supportive treatment should be initiated according to the patient's clinical signs and symptoms. A period of observation and treatment for these symptoms may be necessary, taking into account the half-life of tirzepatide of approximately 5 days.
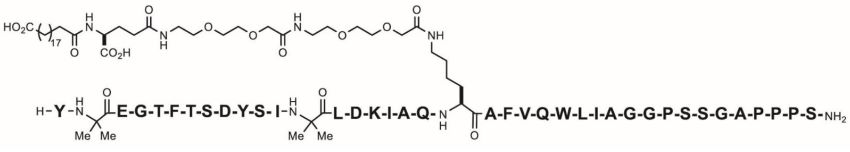
11 DESCRIPTION
ZEPBOUND (tirzepatide) injection, for subcutaneous use, contains tirzepatide, a GIP receptor and GLP-1 receptor agonist. Tirzepatide is based on the GIP sequence and contains aminoisobutyric acid (Aib) in positions 2 and 13, a C-terminal amide, and Lys residue at position 20 that is attached to 1,20-eicosanedioic acid via a linker. The molecular weight is 4813.53 Da and the empirical formula is C225H348N48O68.
Structural formula:
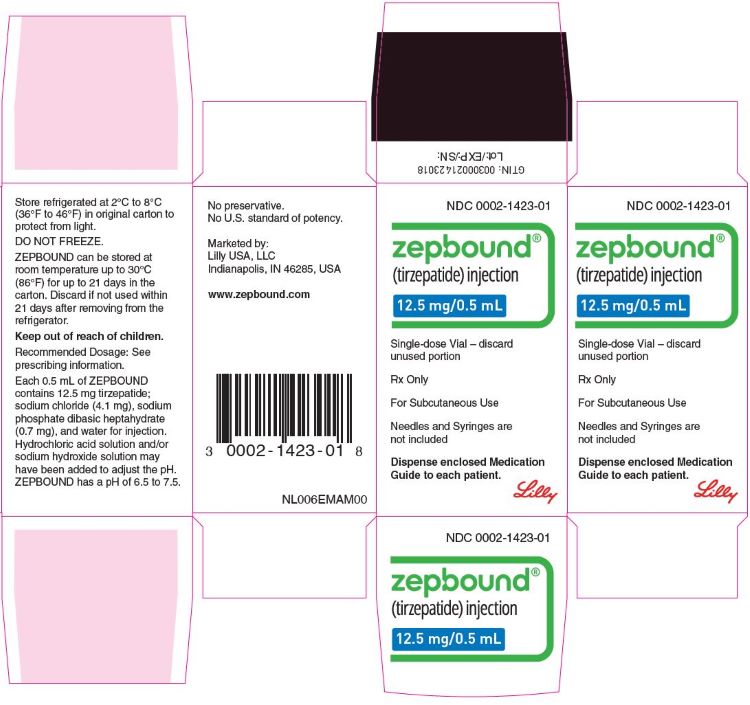
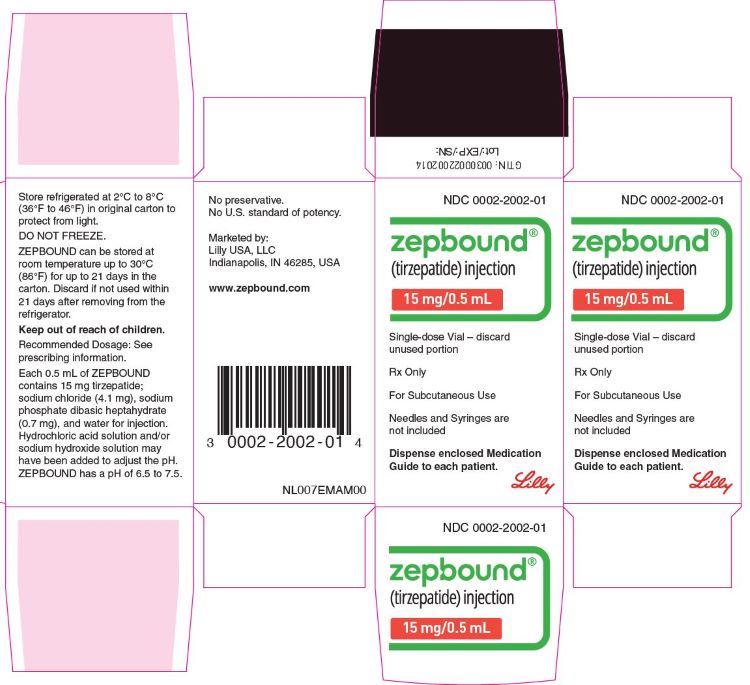
ZEPBOUND is a clear, colorless to slightly yellow, sterile solution for subcutaneous use. Each single-dose pen or single-dose vial contains preservative-free 0.5 mL solution of 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, or 15 mg of tirzepatide and the following excipients: sodium chloride (4.1 mg), sodium phosphate dibasic heptahydrate (0.7 mg), and water for injection. Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH. ZEPBOUND has a pH of 6.5 – 7.5.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tirzepatide is a GIP receptor and GLP-1 receptor agonist. It contains a C20 fatty diacid that enables albumin binding and prolongs the half-life. Tirzepatide selectively binds to and activates both the GIP and GLP-1 receptors, the targets for native GIP and GLP-1.
GLP-1 is a physiological regulator of appetite and caloric intake. Nonclinical studies suggest the addition of GIP may further contribute to the regulation of food intake.
Both GIP receptors and GLP-1 receptors are found in areas of the brain involved in appetite regulation. Animal studies show that tirzepatide distributes to and activates neurons in brain regions involved in regulation of appetite and food intake.
12.2 Pharmacodynamics
Tirzepatide lowers body weight with greater fat mass loss than lean mass loss.
Tirzepatide decreases calorie intake. The effects are likely mediated by affecting appetite.
Tirzepatide stimulates insulin secretion in a glucose-dependent manner and reduces glucagon secretion. Tirzepatide increases insulin sensitivity, as demonstrated in a hyperinsulinemic euglycemic clamp study in patients with type 2 diabetes mellitus after 28 weeks of treatment. These effects can lead to a reduction of blood glucose.
Tirzepatide delays gastric emptying. The delay is largest after the first dose and this effect diminishes over time.
12.3 Pharmacokinetics
The pharmacokinetics of tirzepatide is similar between healthy subjects and patients with overweight or obesity. Steady-state plasma tirzepatide concentrations were achieved following 4 weeks of once weekly administration. Tirzepatide exposure increases in a dose-proportional manner.
Absorption
Following subcutaneous administration, the median time (range) to maximum plasma concentration of tirzepatide is 24 hours (8 to 72 hours). The mean absolute bioavailability of tirzepatide following subcutaneous administration is 80%. Similar exposure was achieved with subcutaneous administration of tirzepatide in the abdomen, thigh, or upper arm.
Distribution
The mean [coefficient of variation (CV)%] apparent steady-state volume of distribution of tirzepatide following subcutaneous administration in patients with overweight or obesity is approximately 9.7 L (28.5%). Tirzepatide is highly bound to plasma albumin (99%).
Elimination
The apparent population mean clearance (CV%) of tirzepatide in patients with overweight or obesity is 0.056 L/h (20.9%) with an elimination half-life of approximately 5 days.
Specific Populations
The intrinsic factors of age (18 to 84 years), sex, race (71% White, 11% Asian, 9% American Indian or Alaska Native, and 8% Black or African American), ethnicity, or body weight do not have a clinically relevant effect on the PK of tirzepatide.
Patients with Renal Impairment
Renal impairment does not impact the pharmacokinetics of tirzepatide. The pharmacokinetics of tirzepatide after a single 5 mg dose were evaluated in patients with different degrees of renal impairment (mild, moderate, severe, ESRD) compared with subjects with normal renal function. Data from clinical studies have also shown that renal impairment in patients with overweight or obesity does not impact the pharmacokinetics of tirzepatide [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Hepatic impairment does not impact the pharmacokinetics of tirzepatide. The pharmacokinetics of tirzepatide after a single 5 mg dose were evaluated in patients with different degrees of hepatic impairment (mild, moderate, severe) compared with subjects with normal hepatic function [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Potential for Tirzepatide to Influence the Pharmacokinetics of Other Drugs
In vitro studies have shown low potential for tirzepatide to inhibit or induce CYP enzymes, and to inhibit drug transporters.
ZEPBOUND delays gastric emptying and thereby has the potential to impact the absorption of concomitantly administered oral medications [see Drug Interactions (7.2)].
The impact of tirzepatide on gastric emptying was greatest after a single dose of 5 mg and diminished after subsequent doses.
Following a first dose of tirzepatide 5 mg, acetaminophen maximum concentration (Cmax) was reduced by 55%, and the median peak plasma concentration (tmax) occurred 1 hour later. After coadministration at Week 6 with tirzepatide 15 mg, there was no meaningful impact on acetaminophen Cmax and tmax. Overall acetaminophen exposure (AUC0-24hr) was not influenced.
Following administration of a combined oral contraceptive (0.035 mg ethinyl estradiol and 0.25 mg norgestimate) in the presence of a single dose of tirzepatide 5 mg, mean Cmax of ethinyl estradiol, norgestimate, and norelgestromin was reduced by 59%, 66%, and 55%, while mean AUC was reduced by 20%, 21%, and 23%, respectively. A delay in tmax of 2.5 to 4.5 hours was observed.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the trials described below with the incidence of anti-drug antibodies in other trials.
During the 72-week treatment period with anti-drug antibodies (ADA) sampling in Studies 1 and 2 [see Clinical Studies (14)], 64.5% (1591/2467) of ZEPBOUND-treated patients developed anti-tirzepatide antibodies. In these trials, anti-tirzepatide antibody formation in 40% and 16.5% of ZEPBOUND-treated patients showed cross-reactivity to native GIP or native GLP-1, respectively.
Of the ZEPBOUND-treated patients, 2.8% and 2.7% developed neutralizing antibodies against tirzepatide activity on the GIP or GLP-1 receptors, respectively, and 0.8% and 0.1% developed neutralizing antibodies against native GIP or GLP-1, respectively.
There was no identified clinically significant effect of anti-tirzepatide antibodies on pharmacokinetics or effectiveness of ZEPBOUND. More ZEPBOUND-treated patients who developed anti-tirzepatide antibodies experienced hypersensitivity reactions or injection site reactions than those who did not develop these antibodies [see Adverse Reactions (6.1)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year carcinogenicity study was conducted with tirzepatide in male and female rats at doses of 0.15, 0.50, and 1.5 mg/kg (0.1-, 0.4-, and 1-fold the MRHD of 15 mg once weekly based on AUC) administered by subcutaneous injection twice weekly. A statistically significant increase in thyroid C-cell adenomas was observed in male rats (≥0.5 mg/kg) and female rats (≥0.15 mg/kg), and a statistically significant increase in thyroid C-cell adenomas and carcinomas combined was observed in male and female rats at all doses examined. In a 6-month carcinogenicity study in rasH2 transgenic mice, tirzepatide at doses of 1, 3, and 10 mg/kg administered by subcutaneous injection twice weekly was not tumorigenic.
Tirzepatide was not genotoxic in a rat bone marrow micronucleus assay.
In fertility and early embryonic development studies, male and female rats were administered twice weekly subcutaneous doses of 0.5, 1.5, or 3 mg/kg (0.3-, 1-, and 2-fold and 0.3-, 0.9-, and 2-fold, respectively, the MRHD of 15 mg once weekly based on AUC). No effects of tirzepatide were observed on sperm morphology, mating, fertility, and conception. In female rats, an increase in the number of females with prolonged diestrus and a decrease in the mean number of corpora lutea resulting in a decrease in the mean number of implantation sites and viable embryos was observed at all dose levels. These effects were considered secondary to the pharmacological effects of tirzepatide on food consumption and body weight.
14 CLINICAL STUDIES
14.1 Weight Reduction and Long-term Maintenance Studies in Adults with Obesity or Overweight
Weight Reduction in Adults with Obesity or Overweight, with or without Type 2 Diabetes Mellitus (Study 1 and Study 2)
Overview of Study 1 and Study 2
The efficacy of ZEPBOUND for weight reduction in conjunction with a reduced-calorie diet and increased physical activity was studied in two randomized, double-blind, placebo-controlled fixed-dosage trials (Study 1 and Study 2) in adults aged 18 years and older. In Studies 1 and 2, all patients received a standard lifestyle intervention which included instruction on a reduced-calorie diet (approximately 500 kcal/day deficit) and increased physical activity counseling (recommended minimum of 150 min/week) that began with the first dose of study medication or placebo and continued throughout the trial. Patients also received counseling on behavior modification strategies to adhere to diet and exercise recommendations. In both trials, weight reduction was assessed after 72 weeks of treatment (at least 52 weeks at maintenance dose).
Study 1 (NCT04184622) was a 72-week trial that enrolled 2,539 adult patients with obesity (BMI ≥30 kg/m2), or with overweight (BMI 27 to <30 kg/m2) and at least one weight-related comorbid condition, such as dyslipidemia, hypertension, obstructive sleep apnea, or cardiovascular disease; patients with type 2 diabetes mellitus were excluded. Patients were randomized in a 1:1:1:1 ratio to once weekly fixed dosage of ZEPBOUND 5 mg, ZEPBOUND 10 mg, ZEPBOUND 15 mg, or placebo, with an escalation period of up to 20 weeks followed by the maintenance period. At baseline, mean age was 45 years (range 18-84 years), 68% were female, 71% were White, 11% were Asian, 9% were American Indian/Alaska Native, and 8% were Black or African American. A total of 48% were Hispanic or Latino ethnicity. Mean baseline body weight was 104.8 kg and mean BMI was 38 kg/m2. Baseline characteristics included 32% with hypertension, 30% with dyslipidemia, 8% with obstructive sleep apnea, and 3% with cardiovascular disease.
Study 2 (NCT04657003) was a 72-week trial that enrolled 938 adult patients with BMI ≥27 kg/m2 and type 2 diabetes mellitus. Patients included in the trial had HbA1c 7-10% and were treated with either diet and exercise alone, or any oral anti-hyperglycemic agent except dipeptidyl peptidase-4 (DPP-4) inhibitors or GLP-1 receptor agonists. Patients who were taking insulin or injectable GLP-1 receptor agonists for type 2 diabetes mellitus were excluded. Patients were randomized in a 1:1:1 ratio to once weekly fixed dosage of ZEPBOUND 10 mg, ZEPBOUND 15 mg, or placebo with an escalation period of up to 20 weeks followed by the maintenance period. At baseline, mean age was 54 years (range 18-85 years), 51% were female, 76% were White, 13% were Asian, and 8% were Black or African American. A total of 60% were Hispanic or Latino ethnicity. Mean baseline body weight was 100.7 kg and mean BMI was 36.1 kg/m2. Baseline characteristics included 66% with hypertension, 61% with dyslipidemia, 8% with obstructive sleep apnea, and 10% with cardiovascular disease.
Results for Study 1 and Study 2
The proportions of patients who discontinued study drug in Study 1 were 14.3%, 16.4%, and 15.1% for the 5 mg, 10 mg, and 15 mg ZEPBOUND-treated groups, respectively, and 26.4% for the placebo-treated group. The proportions of patients who discontinued study drug in Study 2 were 9.3% and 13.8% for the 10 mg and 15 mg ZEPBOUND-treated groups, respectively, and 14.9% for the placebo-treated group.
For Studies 1 and 2, weight reduction was assessed after 72 weeks of treatment (at least 52 weeks at maintenance dose). In both studies, the primary efficacy parameters were mean percent change in body weight and the percentage of patients achieving ≥5% weight reduction from baseline to Week 72 (see Table 2).
After 72 weeks of treatment, ZEPBOUND resulted in a statistically significant reduction in body weight compared with placebo, and greater proportions of patients treated with ZEPBOUND 5 mg, 10 mg, and 15 mg achieved at least 5% weight reduction compared to placebo. Among patients treated with ZEPBOUND 10 mg and 15 mg, greater proportions of patients achieved at least 10%, 15%, and 20% weight reduction compared to placebo (see Table 2). A reduction in body weight was observed with ZEPBOUND irrespective of age, sex, race, ethnicity, baseline BMI, and glycemic status.
|
Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; N = number of patients randomly assigned to study drug. |
|||||||
|
a The intention-to-treat population includes all randomly assigned patients. For Study 1 at Week 72, body weight was missing for 21.6%, 10.2%, 10.5%, and 9.4% of patients randomly assigned to placebo, ZEPBOUND 5 mg, 10 mg, and 15 mg, respectively. For Study 2 at Week 72, body weight was missing for 11.1%, 4.8%, and 8.4% of patients randomly assigned to placebo, ZEPBOUND 10 mg, and 15 mg, respectively. The missing values were imputed by a hybrid approach using retrieved dropouts from the same treatment group (if missing not due to COVID-19) or using all non-missing data assuming missing at random (for missing solely due to COVID-19). |
|||||||
|
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. |
|||||||
|
c Analyzed using logistic regression adjusted for baseline value. |
|||||||
|
d p-value<0.001 (unadjusted 2-sided) for superiority, controlled for type I error rate. |
|||||||
|
e Not controlled for type I error rate. |
|||||||
| Study 1 | Study 2 | ||||||
| Intention-to-Treat (ITT) Populationa | Placebo N = 643 |
ZEPBOUND 5 mg N = 630 |
ZEPBOUND 10 mg N = 636 |
ZEPBOUND 15 mg N = 630 |
Placebo N = 315 |
ZEPBOUND 10 mg N = 312 |
ZEPBOUND 15 mg N = 311 |
| Body Weight | |||||||
| Baseline mean (kg) | 104.8 | 102.9 | 105.8 | 105.6 | 101.7 | 100.9 | 99.6 |
| % Change from baselineb | -3.1 | -15.0 | -19.5 | -20.9 | -3.2 | -12.8 | -14.7 |
| % Difference from placebob (95% CI) |
-11.9 (-13.4, _10.4)d |
-16.4 (_17.9, -14.8)d |
-17.8 (_19.3, -16.3)d |
-9.6 (-11.1, -8.1)d |
-11.6 (-13.0, -10.1)d |
||
| % of Patients losing ≥5% body weight | 34.5 | 85.1 | 88.9 | 90.9 | 32.5 | 79.2 | 82.8 |
| % Difference from placebo (95% CI) |
50.3 (44.3, 56.2)c,d |
54.6 (49.1, 60.0)c,d |
56.4 (50.9, 62.0)c,d |
46.8 (39.5, 54.1)c,d |
50.4 (43.1, 57.8)c,d |
||
| % of Patients losing ≥10% body weight | 18.8 | 68.5 | 78.1 | 83.5 | 9.5 | 60.5 | 64.8 |
| % Difference from placebo (95% CI) |
49.3 (43.6, 54.9)c,e |
59.5 (54.2, 64.9)c,d |
64.8 (59.6, 70.1)c,d |
51.0 (44.4, 57.7)c,d |
55.3 (48.6, 62.0)c,d |
||
| % of Patients losing ≥15% body weight | 8.8 | 48.0 | 66.6 | 70.6 | 2.7 | 39.7 | 48.0 |
| % Difference from placebo (95% CI) |
38.7 (33.6, 43.7)c,e |
58.1 (53.2, 63.0)c,d |
62.0 (57.2, 66.8)c,d |
37.0 (31.1, 42.9)c,d |
45.4 (39.4, 51.4)c,d |
||
| % of Patients losing ≥20% body weight | 3.1 | 30.0 | 50.1 | 56.7 | 1.0 | 21.5 | 30.8 |
| % Difference from placebo (95% CI) |
26.6 (22.4, 30.7)c,e |
47.3 (42.7, 51.9)c,d |
53.8 (49.3, 58.3)c,d |
20.5 (15.7, 25.4)c,d |
29.7 (24.3, 35.0)c,d |
||
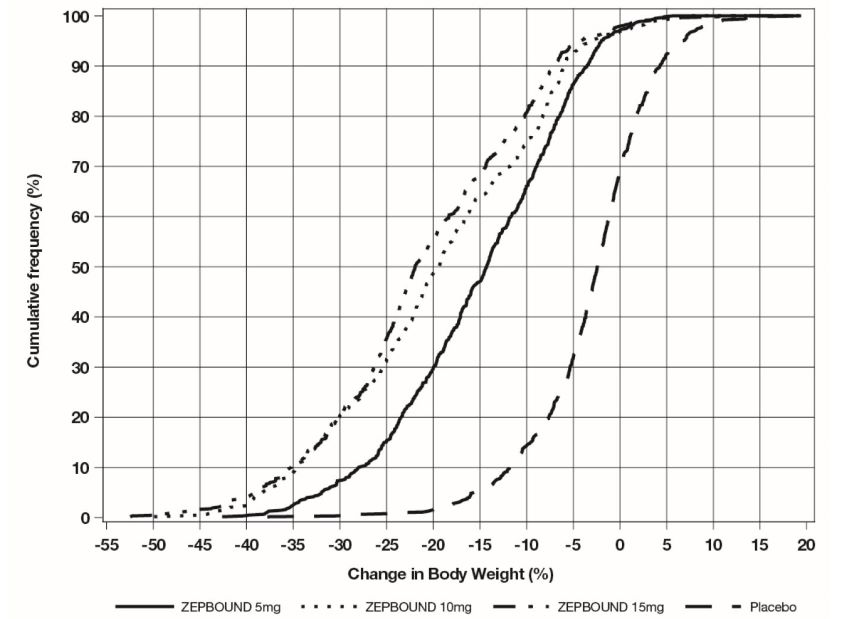
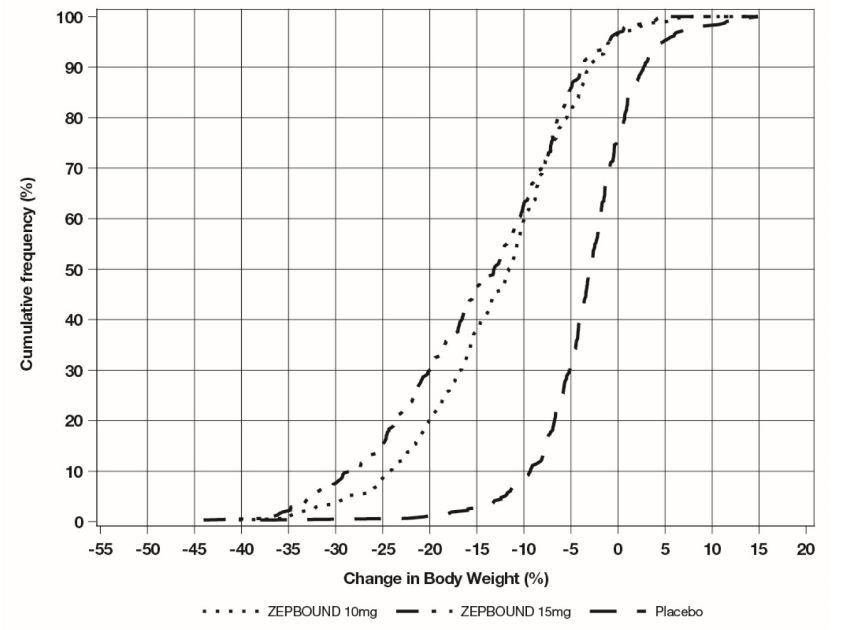
The cumulative frequency distributions of change in body weight are shown in Figure 1 for Study 1 and Figure 2 for Study 2. One way to interpret this figure is to select a change in body weight
of interest on the horizontal axis and note the corresponding proportions of patients
(vertical axis) in each treatment group who achieved at least that degree of weight
reduction. For example, note that the vertical line arising from -10% in Figure 1 intersects the ZEPBOUND 15 mg and placebo curves at approximately 83.5%, and 18.8%,
respectively, which correspond to the values shown in Table 2.
Figure 1: Changes in Body Weight (%) from Baseline to Week 72 in Study 1 in Patients with Obesity or Overweight (without Type 2 Diabetes)
Note: Based on average percent weight change of each randomized patient within each
specific treatment arm from 100 imputed datasets including observed data and imputed
data using hybrid approach for missing values.
Figure 2: Changes in Body Weight (%) from Baseline to Week 72 in Study 2 in Patients with Obesity or Overweight and Type 2 Diabetes
Note: Based on average percent weight change of each randomized patient within each specific treatment arm from 100 imputed datasets including observed data and imputed data using hybrid approach for missing values.
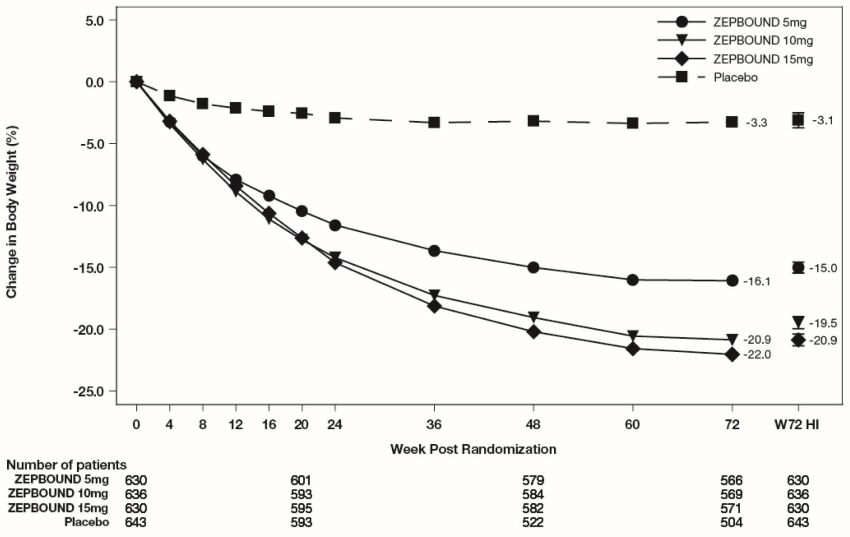
The time courses of weight reduction with ZEPBOUND and placebo from baseline through
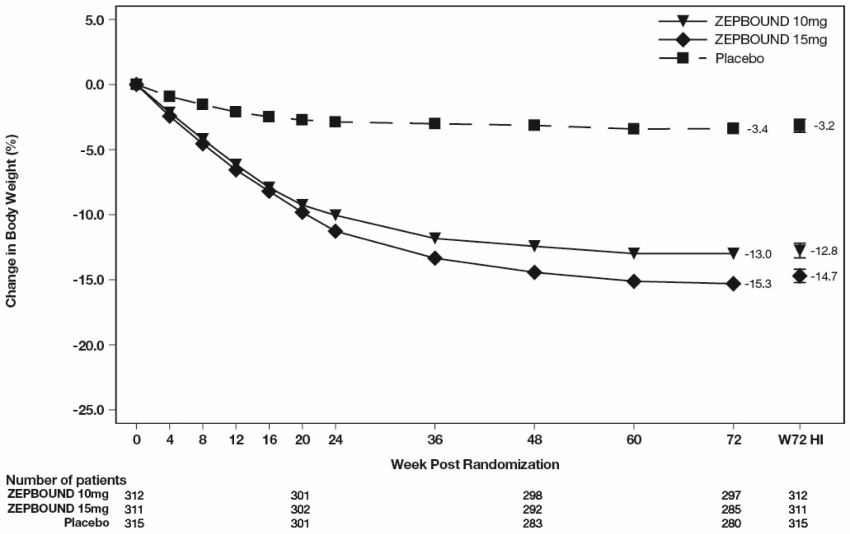
Week 72 are depicted in Figure 3 for Study 1 and Figure 4 for Study 2.
Figure 3: Change from Baseline (%) in Body Weight in Study 1 in Patients with Obesity or Overweight (without Type 2 Diabetes)
Note: Displayed results are from the Intent-to-Treat Population. (1) Observed mean
value from Week 0 to Week 72, and (2) least-squares mean ± standard error at Week
72 hybrid imputation (HI).
Figure 4: Change from Baseline (%) in Body Weight in Study 2 in Patients with Obesity or Overweight and Type 2 Diabetes
Note: Displayed results are from the Intent-to-Treat Population. (1) Observed mean value from Week 0 to Week 72, and (2) least squares mean ± standard error at Week 72 hybrid imputation (HI).
Changes in waist circumference and cardiometabolic parameters with ZEPBOUND are shown in Table 3 for Study 1 and Study 2.
|
Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; N = number of patients randomly assigned to study drug. |
|||||||
|
a The intention-to-treat population includes all randomly assigned patients. The missing values were imputed by a hybrid approach using retrieved dropouts from the same treatment group (if missing not due to COVID-19) or using all non-missing data assuming missing at random (for missing solely due to COVID-19). |
|||||||
|
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. |
|||||||
|
c Analyzed using log-transformed data. |
|||||||
|
d p-value<0.001 (unadjusted 2-sided) for superiority, controlled for type I error rate. |
|||||||
|
e Not controlled for type I error rate. |
|||||||
|
f Least-squares mean from mixed model for repeated measures adjusted for baseline value and other stratification factors. |
|||||||
|
g Baseline value is the geometric mean. |
|||||||
| Study 1 | Study 2 | ||||||
| Intention-to-Treat (ITT) Populationa | Placebo N = 643 |
ZEPBOUND 5 mg N = 630 |
ZEPBOUND 10 mg N = 636 |
ZEPBOUND 15 mg N = 630 |
Placebo N = 315 |
ZEPBOUND 10 mg N = 312 |
ZEPBOUND 15 mg N = 311 |
| Waist Circumference (cm) | |||||||
| Baseline mean | 114.0 | 113.2 | 114.8 | 114.4 | 116.0 | 114.2 | 114.6 |
| Change from baselineb | -4.0 | -14.0 | -17.7 | -18.5 | -3.3 | -10.8 | -13.1 |
| Difference from placebob (95% CI) | -10.1 (-11.6, -8.6)e |
-13.8 (-15.2, -12.3)d |
-14.5 (-15.9, -13.0)d |
-7.4 (-9.0, -5.9)d |
-9.8 (-11.2, -8.3)d |
||
| Systolic Blood Pressure (mmHg) | |||||||
| Baseline mean | 122.9 | 123.6 | 123.8 | 123.0 | 131.0 | 130.6 | 130.0 |
| Change from baselineb | -1.0 | -6.6 | -7.7 | -7.4 | -1.2 | -5.6 | -7.1 |
| Difference from placebob (95% CI) | -5.6 (-7.2, -3.9)e |
-6.7 (-8.4, -5.0)e |
-6.4 (-8.0, -4.8)e |
-4.4 (-6.7, -2.1)e |
-5.9 (-8.3, -3.6)e |
||
| Diastolic Blood Pressure (mmHg) | |||||||
| Baseline mean | 79.6 | 79.3 | 79.9 | 79.3 | 79.4 | 80.2 | 79.7 |
| Change from baselineb | -0.8 | -4.9 | -5.0 | -4.5 | -0.3 | -2.1 | -2.9 |
| Difference from placebob (95% CI) | -4.1 (-5.2, -3.0)e |
-4.2 (-5.3, -3.0)e |
-3.7 (-4.8, -2.7)e |
-1.8 (-3.3, -0.4)e |
-2.7 (-4.2, -1.2)e |
||
| Pulse Rate (beats per minute) | |||||||
| Baseline mean | 72.9 | 72.4 | 71.8 | 72.4 | 74.8 | 75.9 | 75.6 |
| Change from baselinef | 0.1 | 0.6 | 2.3 | 2.6 | -0.5 | 0.6 | 1.0 |
| Difference from placebof (95% CI) | 0.5 (-0.5, 1.5)e |
2.2 (1.2, 3.2)e |
2.5 (1.5, 3.4)e |
1.2 (-0.1, 2.5)e |
1.5 (0.2, 2.8)e |
||
| Total Cholesterol (mg/dL) | |||||||
| Baseline meang | 187.5 | 187.1 | 190.6 | 187.5 | 174.9 | 173.9 | 167.0 |
| % change from baselineb | -1.8 | -3.8 | -4.4 | -6.3 | 2.8 | -2.8 | -1.0 |
| Relative difference from placebob (95% CI) | -2.1 (-4.5, 0.4)c,e |
-2.7 (-5.1, -0.2)c,e |
-4.6 (-6.8, -2.2)c,e |
-5.5 (-8.7, -2.2)c,e |
-3.8 (-7.1, -0.3)c,e |
||
| LDL Cholesterol (mg/dL) | |||||||
| Baseline meang | 109.4 | 108.7 | 112.3 | 109.3 | 92.4 | 90.5 | 85.7 |
| % change from baselineb | -1.7 | -4.6 | -5.6 | -7.1 | 7.4 | 1.8 | 4.1 |
| Relative difference from placebob (95% CI) | -2.9 (-6.6, 0.9)c,e |
-4.0 (-7.5, -0.5)c,e |
-5.5 (-8.9, -2.0)c,e |
-5.2 (-10.1, 0.1)c,e |
-3.0 (-8.4, 2.6)c,e |
||
| HDL Cholesterol (mg/dL) | |||||||
| Baseline meang | 46.6 | 47.6 | 47.6 | 47.6 | 42.7 | 43.8 | 42.2 |
| % change from baselineb | -0.7 | 6.9 | 9.2 | 8.0 | 0.2 | 8.2 | 9.7 |
| Relative difference from placebob (95% CI) | 7.7 (4.6, 10.8)c,e |
9.9 (6.7, 13.2)c,e |
8.7 (5.7, 11.8)c,e |
8.0 (4.2, 11.8)c,e |
9.5 (5.6, 13.5)c,e |
||
| Non-HDL Cholesterol (mg/dL) | |||||||
| Baseline meang | 138.3 | 137.0 | 140.4 | 137.5 | 129.6 | 127.2 | 121.9 |
| % change from baselineb | -2.3 | -8.0 | -9.4 | -11.7 | 3.7 | -6.6 | -5.2 |
| Relative difference from placebob (95% CI) | -5.8 (-8.9, -2.6)c,e |
-7.2 (-10.3, -4.1)c,e |
-9.6 (-12.4, -6.6)c,e |
-9.9 (-14.1, -5.6)c,e |
-8.5 (-12.9, -4.0)c,e |
||
| Triglycerides (mg/dL) | |||||||
| Baseline meang | 130.8 | 128.7 | 125.7 | 128.1 | 165.0 | 158.8 | 158.5 |
| % change from baselineb | -5.6 | -21.2 | -23.8 | -29.1 | -3.3 | -27.1 | -27.3 |
| Relative difference from placebob (95% CI) | -16.5 (-21.2, -11.4)c,e |
-19.3 (-23.9, -14.4)c,e |
-24.9 (-29.1, -20.4)c,e |
-24.6 (-30.0, -18.7)c,e |
-24.8 (-30.3, -18.9)c,e |
||
| HbA1c (%) | |||||||
| Baseline mean | 5.6 | 5.6 | 5.5 | 5.6 | 8.0 | 8.0 | 8.1 |
| Change from baselineb | -0.1 | -0.4 | -0.4 | -0.4 | -0.5 | -2.1 | -2.1 |
| Difference from placebob (95% CI) | -0.3 (-0.3, -0.2)e |
-0.4 (-0.4, -0.3)e |
-0.4 (-0.4, -0.3)e |
-1.6 (-1.7, -1.4)d |
-1.6 (-1.8, -1.4)d |
||
Weight Reduction Following Intensive Lifestyle Intervention in Adults with Obesity or Overweight (Study 3)
Overview of Study 3
Study 3 (NCT04657016) was an 84-week trial with a 12-week intensive lifestyle intervention lead-in period (Week -12 to Week 0), followed by a 72-week randomized treatment period of ZEPBOUND versus placebo (Week 0 to Week 72) with a standard lifestyle intervention. Only patients who lost ≥5% body weight during the 12-week intensive lifestyle lead-in period entered the 72-week randomized treatment period. The trial initially enrolled 806 adult patients (aged 18 years and older) with obesity (BMI ≥30 kg/m2), or with overweight (BMI 27 to <30 kg/m2) and at least one weight-related comorbid condition, such as dyslipidemia, hypertension, obstructive sleep apnea, or cardiovascular disease; patients with type 2 diabetes mellitus were excluded. During the intensive lifestyle intervention lead-in period, lifestyle instruction was delivered 8 times over 12 weeks by a dietician or dietician-equivalent, with all patients receiving instruction to exercise for at least 150 minutes per week and to reduce their caloric intake to approximately 1,200 kcal/day (females) or 1,500 kcal/day (males). Patients also received counseling on behavior modification strategies to adhere to diet and exercise recommendations. At the end of the 12-week intensive lifestyle intervention lead-in period, 579 patients who achieved ≥5% weight reduction were randomized in a 1:1 ratio to ZEPBOUND or placebo for 72 weeks. ZEPBOUND dosages were escalated over a period of up to 20 weeks to a maximum tolerated dosage (MTD) of 10 mg or 15 mg subcutaneous once weekly. During the randomized treatment period, patients received a standard lifestyle instruction every 12 weeks on reduced-calorie diet (approximately 500 kcal/day deficit) and increased physical activity (recommended minimum of 150 min/week) that began with the first dose of ZEPBOUND or placebo and continued throughout the 72-week treatment period; behavior modification strategies were recommended as needed. Weight reduction was assessed after 72 weeks of treatment (at least 52 weeks at maintenance dose).
For the 579 patients who were randomized, mean body weight at enrollment prior to entering the 12-week lifestyle lead-in period (Week -12) was 109.5 kg and mean BMI was 38.6 kg/m2. At randomization (Week 0), after the 12-week intensive lifestyle lead-in period, mean body weight was 101.9 kg and mean BMI was 35.9 kg/m2. The mean age of patients randomized to treatment was 46 years (range 18-77 years), 63% were female, 86% were White, 11% were Black or African American, and 1% were Asian. A total of 54% were Hispanic or Latino ethnicity. Baseline characteristics for the 579 randomized patients included 34% with hypertension, 26% with dyslipidemia, 10% with obstructive sleep apnea, and 2% with cardiovascular disease.
Results for Study 3
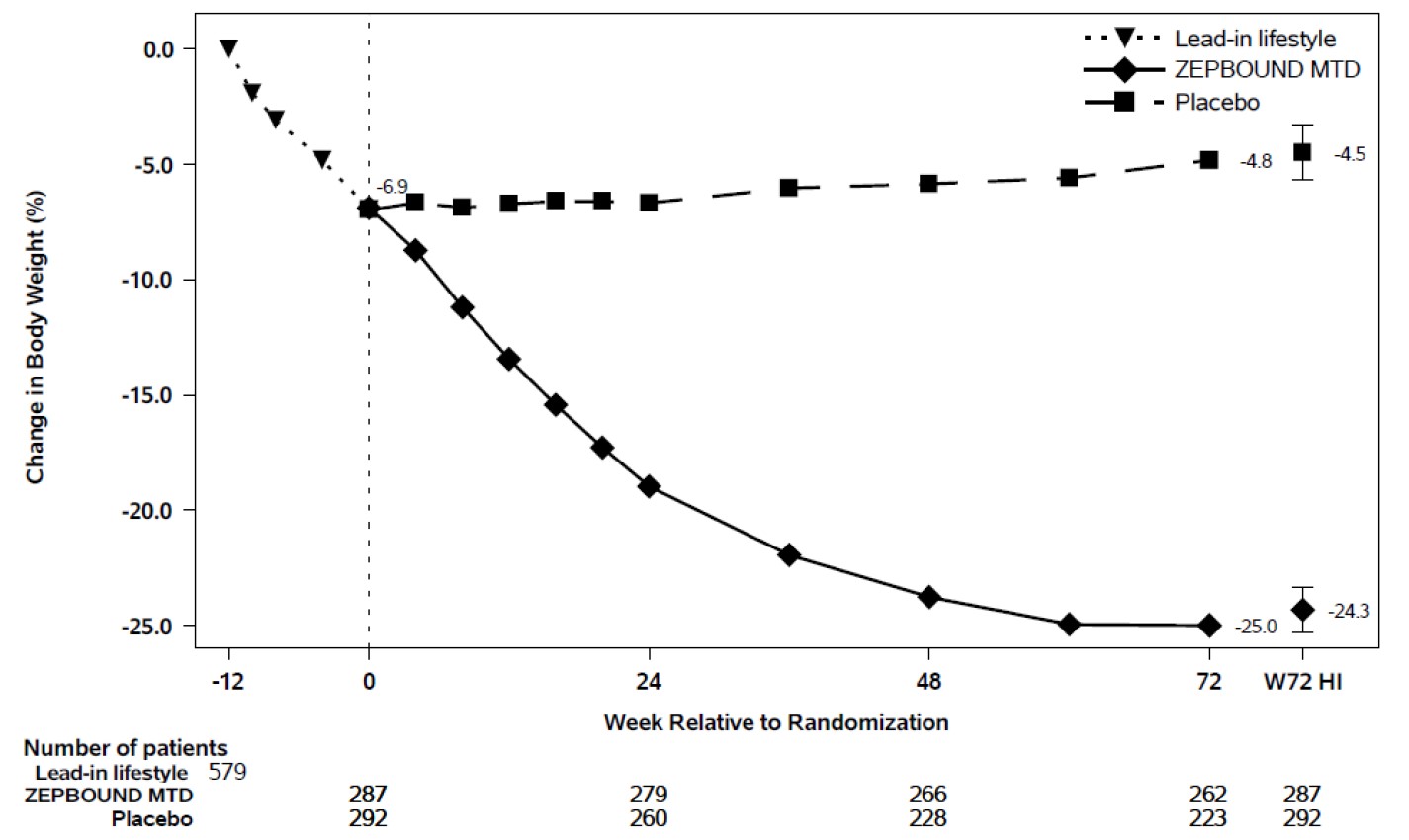
At the end of the 12-week intensive lifestyle intervention lead-in, for patients who
subsequently entered the randomized treatment period (n=579), the average body weight
loss due to lifestyle was 6.9% (Week -12 to Week 0). Eighty-six percent (86%) of ZEPBOUND-treated
patients had a maximum tolerated dosage of 15 mg weekly based on their final dose
during the double-blind treatment period. The time course of weight reduction during
the lead-in and from Week 0 to Week 72 with ZEPBOUND and placebo are depicted in Figure 5.
Figure 5: Change in Body Weight (%) After 12-Week Intensive Lifestyle Intervention Lead-In Followed by Randomized Treatment and a Standard Lifestyle Intervention (Study 3) in Patients with Obesity or Overweight
Note: Displayed results are from the randomized Population. (1) Observed mean value from Week -12 to Week 72, and (2) least squares mean ± standard error at Week 72 hybrid imputation (HI). Change from Week -12 is not a primary endpoint in Study 3.
The proportions of patients who discontinued study drug after randomization were 21.3% for the ZEPBOUND-treated group and 30.5% for the placebo-treated group.
For Study 3, the primary efficacy parameters were mean percent change in body weight from randomization (Week 0) to Week 72 and the percentage of patients achieving ≥5% weight reduction from randomization (Week 0) to Week 72. Amongst randomized patients who already lost ≥5% body weight during the 12-week intensive lifestyle lead-in period, subsequent treatment with ZEPBOUND resulted in a statistically significant reduction in body weight compared to placebo from randomization (Week 0) to Week 72. A greater proportion of patients treated with ZEPBOUND achieved at least 5%, 10%, 15%, and 20% weight reduction from Week 0 to Week 72 compared to placebo (see Table 4).
|
Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; MTD = maximum tolerated dose; N = number of patients randomly assigned to study drug. |
||
|
a The intent-to-treat population included only randomized patients with ≥5% weight loss at Week 0 after 12 weeks of intensive lifestyle intervention. During the 12-week lead-in period, 227 of 806 patients (28.2%) discontinued from the study. Of these 141 (17.5%) discontinued due to not achieving the randomization criteria of ≥5% weight reduction. |
||
|
b The intent-to-treat population includes all randomly assigned patients. For Study 3 at Week 72, body weight was missing for 23.6% and 8.7% of patients randomly assigned to placebo and ZEPBOUND MTD (10 or 15 mg). The missing values were imputed by a hybrid approach using retrieved dropouts from the same treatment group (if missing not due to COVID-19) or using all non-missing data assuming missing at random (for missing solely due to COVID-19). |
||
|
c Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. |
||
|
d Analyzed using logistic regression adjusted for baseline value. |
||
|
e p-value<0.001 (unadjusted 2-sided) for superiority, controlled for type I error rate. |
||
| Study 3 N = 579a |
||
| Body weight | ||
| Mean (kg) at Week -12 | 109.5 | |
| Intention-to-Treat (ITT) Populationa,b | Placebo N = 292 |
ZEPBOUND MTD (10 mg or 15 mg) N = 287 |
| Body Weight | ||
| Mean (kg) at Week 0 | 101.3 | 102.5 |
| % Change from randomization at Week 72c | 2.5 | -18.4 |
| % Difference from placebo, at Week 72c (95% CI) |
-20.8 (-23.2, -18.5)e |
|
| % of Patients losing ≥5% body weight | 16.5 | 87.5 |
| % Difference from placebo (95% CI) |
71.1 (63.6, 78.5)d,e |
|
| % of Patients losing ≥10% body weight | 8.9 | 76.7 |
| % Difference from placebo (95% CI) |
67.9 (60.7, 75.1)d,e |
|
| % of Patients losing ≥15% body weight | 4.2 | 65.4 |
| % Difference from placebo (95% CI) |
61.3 (54.5, 68.1)d,e |
|
| % of Patients losing ≥20% body weight | 2.2 | 44.7 |
| % Difference from placebo (95% CI) |
42.6 (36.0, 49.1)d,e |
|
Changes in waist circumference and cardiometabolic parameters are shown in Table 5.
|
Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; N = number of patients randomly assigned to study drug. |
|||||||
|
a The intent-to-treat population included all randomly assigned patients. The missing values were imputed by a hybrid approach using retrieved dropouts from the same treatment group (if missing not due to COVID-19) or using all non-missing data assuming missing at random (for missing solely due to COVID-19). |
|||||||
|
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. |
|||||||
|
c Analyzed using log-transformed data. |
|||||||
|
d p-value<0.001 (unadjusted 2-sided) for superiority, controlled for type I error rate. |
|||||||
|
e Not controlled for type I error rate. |
|||||||
|
f Least-squares mean from mixed model for repeated measures adjusted for baseline value and other stratification factors. |
|||||||
|
g Baseline and randomization values are the geometric mean. |
|||||||
|
h Observed means are shown for change from Week -12 to Week 0. Least-square means are shown for change from Week 0 to Week 72. |
|||||||
| Intention-to-Treat (ITT) Populationa | All Randomized Patients N=579 |
Placebo N=292 |
ZEPBOUND MTD (10 mg or 15 mg) N=287 |
||||
| Baseline (Week -12) | Change from Week -12 to Week 0 | Randomization (Week 0) | Change from Week 0 to Week 72 | Randomization (Week 0) | Change from Week 0 to Week 72 | Difference from placebo, Week 0 to Week 72 (95% CI) |
|
| Waist circumference (cm)h | 116.1 | -6.7 | 109.6 | 0.2b | 109.3 | -14.6b | -14.8b (-17.2, -12.5)d |
| Systolic Blood Pressure (mmHg)h | 126.2 | -5.0 | 120.8 | 3.5b | 121.7 | -5.1b | -8.6b (-11.3, -6.0)e |
| Diastolic Blood Pressure (mmHg)h | 81.7 | -2.9 | 78.3 | 2.1b | 79.3 | -3.2b | -5.3b (-6.9, -3.7)e |
| Pulse Rate (beats per minute)h | 73.0 | -1.6 | 70.7 | 0.9f | 72.2 | 2.7f | 1.8f (0.3, 3.4)e |
| HbA1c (%)h | 5.5 | -0.1 | 5.4 | 0.0b | 5.3 | -0.4b | -0.4b (-0.5, -0.3)e |
| Total Cholesterol (mg/dL)g,h | 190.2 | -8.6 | 181.6 | 4.3 | 181.7 | -2.4 | -6.4b (-9.0, -3.6)c,e |
| LDL Cholesterol (mg/dL)g,h | 111.6 | -3.5 | 107.5 | 4.4 | 108.0 | -5.6 | -9.6b (-13.7, -5.4)c,e |
| HDL Cholesterol (mg/dL)g,h | 48.4 | -1.2 | 47.8 | 5.4 | 46.9 | 15.2 | 9.3b (4.5, 14.2)c,e |
| Non-HDL Cholesterol (mg/dL)g,h | 139.2 | -7.4 | 131.5 | 4.4 | 132.4 | -8.8 | -12.6b (-15.9, -9.3)c,e |
| Triglycerides (mg/dL)g,h | 123.1 | -19.8 | 108.8 | 2.1 | 111.7 | -23.5 | -25.1b (-30.9, -18.9)c,e |
Weight Reduction Following Randomized Withdrawal in Adults with Obesity or Overweight (Study 4)
Overview of Study 4
Study 4 (NCT04660643) was an 88-week randomized withdrawal trial in which all patients received open-label ZEPBOUND during a 36-week lead-in period, followed by randomization to either continue ZEPBOUND or switch to placebo for 52 weeks. The trial enrolled 783 adult patients (aged 18 years and older) with obesity (BMI ≥30 kg/m2), or with overweight (BMI 27 to <30 kg/m2) and at least one weight-related comorbid condition, such as dyslipidemia, hypertension, obstructive sleep apnea, or cardiovascular disease; patients with type 2 diabetes mellitus were excluded. All patients received a standard lifestyle intervention which included instruction on a reduced-calorie diet (approximately 500 kcal/day deficit) and increased physical activity counseling (recommended minimum of 150 min/week) that began with the first dose of ZEPBOUND, during the lead-in period, and continued throughout the trial. During the 36-week open-label ZEPBOUND lead-in period, ZEPBOUND dosages were escalated over a period of up to 20 weeks to an MTD of 10 mg or 15 mg subcutaneous once weekly. After the lead-in period, patients were randomized at Week 36 to continue ZEPBOUND or switch to placebo for 52 weeks.
Of the 783 patients who started ZEPBOUND at Week 0, 14.4% discontinued treatment before randomization at Week 36, and adverse events were the most common reason for discontinuation (6.8%). At Week 36, a total of 670 patients were randomized in a 1:1 ratio to ZEPBOUND MTD or placebo for 52 weeks. For the 670 randomized patients, at study entry (Week 0) mean body weight was 107.3 kg and mean BMI was 38.4 kg/m2, and at randomization (Week 36, after the open-label ZEPBOUND lead-in period) mean body weight was 85.2 kg and mean BMI was 30.5 kg/m2. Among the randomized patients, the mean age was 49 years (range 19-81 years), 71% were female, 80% were White, 11% were Black or African American, and 7% were Asian. A total of 44% were Hispanic or Latino ethnicity. Baseline characteristics for the 670 randomized patients included 35% with hypertension, 32% with dyslipidemia, 12% with obstructive sleep apnea, and 6% with cardiovascular disease.
Results for Study 4
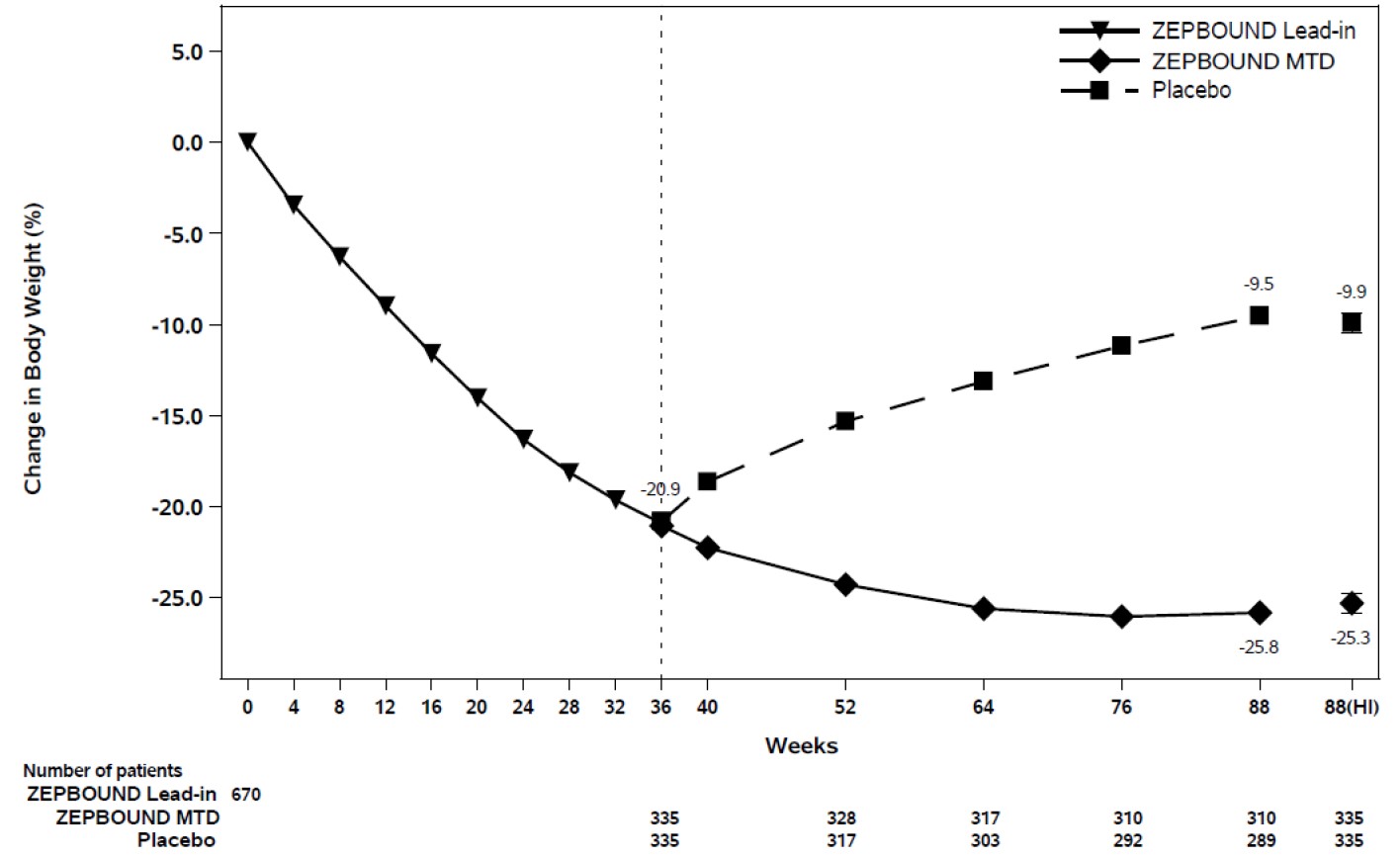
At the end of the 36-week open-label ZEPBOUND lead-in period, of the 670 randomized
patients, 93% were on ZEPBOUND MTD of 15 mg weekly and 7% were on MTD of 10 mg weekly.
After open-label ZEPBOUND treatment, randomized patients (n=670) had an average body
weight loss of 20.9% (Week 0 to Week 36). The time course of weight reduction from
Week 0 through Week 88 is depicted in Figure 6.
Figure 6: Change in Body Weight (%) After 36-Week Open-Label Treatment Followed by Randomized Withdrawal (Study 4) in Patients with Obesity or Overweight
Note: Displayed results are from the randomized population. (1) Displayed results are observed mean value from Week 0 to Week 88 and (2) least squares mean ± standard error at Week 88 hybrid imputation (HI). Change from Week 0 was not a primary endpoint in Study 4.
The proportions of patients who discontinued study drug after randomization at Week 36 were 10.4% for the ZEPBOUND-treated group and 17.9% for the placebo-treated group.
For Study 4, the primary efficacy parameter was mean percent change in body weight from randomization (Week 36) to Week 88. After weight loss with ZEPBOUND treatment during the open-label lead-in period (Week 0 to Week 36), continued treatment with ZEPBOUND from randomization (Week 36) to Week 88 resulted in a statistically significant reduction in body weight compared with placebo (see Table 6).
|
Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; MTD = maximum tolerated dose; N = number of patients randomly assigned to study drug. |
||
|
a The intent-to-treat population included all randomly assigned patients and did not include 113 patients who were enrolled but not randomized. At Week 88, body weight was missing for 13.7% and 7.5% of patients randomly assigned to placebo and ZEPBOUND MTD (10 or 15 mg), respectively. The missing values were imputed by a hybrid approach using retrieved dropouts from the same treatment group (if missing not due to COVID-19) or using all non-missing data assuming missing at random (for missing solely due to COVID-19). |
||
|
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. |
||
|
c Analyzed using logistic regression adjusted for baseline value. |
||
|
d p-value<0.001 (unadjusted 2-sided) for superiority, controlled for type I error rate. |
||
| Study 4 N=670a |
||
| Body weight | ||
| Mean at Week 0 (kg) | 107.3 | |
| Intention-to-Treat (ITT) Populationa | Placebo N=335 |
ZEPBOUND (MTD 10 mg or 15 mg) N=335 |
| Body Weight | ||
| Mean at Week 36 (kg) | 85.8 | 84.6 |
| % change from Week 36 at Week 88b | 14.0 | -5.5 |
| % difference from placebo at Week 88 (95% CI)b |
-19.4 (-21.2, -17.7)d |
|
Changes in waist circumference and cardiometabolic parameters in Study 4 are shown in Table 7.
|
Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; N = number of patients randomly assigned to study drug. |
|||||||
|
a The intent-to-treat population included all randomly assigned patients. The missing values were imputed by a hybrid approach using retrieved dropouts from the same treatment group (if missing not due to COVID-19) or using all non-missing data assuming missing at random (for missing solely due to COVID-19). |
|||||||
|
b Least-squares mean from ANCOVA adjusted for baseline value and other stratification factors. |
|||||||
|
c Analyzed using log-transformed data. |
|||||||
|
d p-value<0.001 (unadjusted 2-sided) for superiority, controlled for type I error rate. |
|||||||
|
e Not controlled for type I error rate. |
|||||||
|
f Least-squares mean from mixed model for repeated measures adjusted for baseline value and other stratification factors. |
|||||||
|
g Baseline and randomization values are the geometric mean. |
|||||||
|
h Observed means are shown for change from Week 0 to Week 36. Least-square means are shown for change from Week 36 to Week 88. |
|||||||
| Intention-to-Treat (ITT) Populationa | All Randomized Patients N=670 |
Placebo N=335 |
ZEPBOUND MTD (10 mg or 15 mg) N=335 |
||||
| Baseline (Week 0) | Change from Week 0 to Week 36 | Randomization (Week 36) | Change from Week 36 to Week 88 | Randomization (Week 36) | Change from Week 36 to Week 88 | Difference from placebo, Week 36 to Week 88 (95% CI) |
|
| Waist circumference (cm)h | 115.2 | -17.8 | 98.2 | 7.8b | 96.8 | -4.3b | -12.1b (-13.5, -10.6)d |
| Systolic Blood Pressure (mmHg)h | 126.1 | -11.2 | 114.8 | 8.2b | 115.0 | 2.0b | -6.2b (-8.2, -4.3)e |
| Diastolic Blood Pressure (mmHg)h | 80.9 | -5.1 | 76.2 | 3.2b | 75.4 | -0.7b | -3.8b (-5.2, -2.4)e |
| Pulse Rate (beats per minute)h | 72.5 | 5.0 | 77.8 | -5.2f | 77.1 | -2.1f | 3.1f (1.9, 4.3)e |
| HbA1c (%)h | 5.5 | -0.5 | 5.0 | 0.3b | 5.1 | -0.0b | -0.3b (-0.3, -0.2)e |
| Total Cholesterol (mg/dL)g,h | 188.3 | -12.4 | 176.1 | 8.0 | 175.9 | 2.7 | -4.9b (-7.4, -2.4)c,e |
| LDL Cholesterol (mg/dL)g,h | 108.6 | -1.9 | 107.6 | 3.2 | 105.9 | -3.5 | -6.5b (-10.0, -2.9)c,e |
| HDL Cholesterol (mg/dL)g,h | 49.9 | -2.7 | 47.3 | 14.8 | 47.7 | 18.7 | 3.4b (0.2, 6.6)c,e |
| Non-HDL Cholesterol (mg/dL)g,h | 135.8 | -9.8 | 126.3 | 5.1 | 126.0 | -3.4 | -8.1b (-11.3, -4.8)c,e |
| Triglycerides (mg/dL)g,h | 121.4 | -40.4 | 85.5 | 13.5 | 90.9 | -4.8 | -16.1b (-21.7, -10.0)c,e |
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
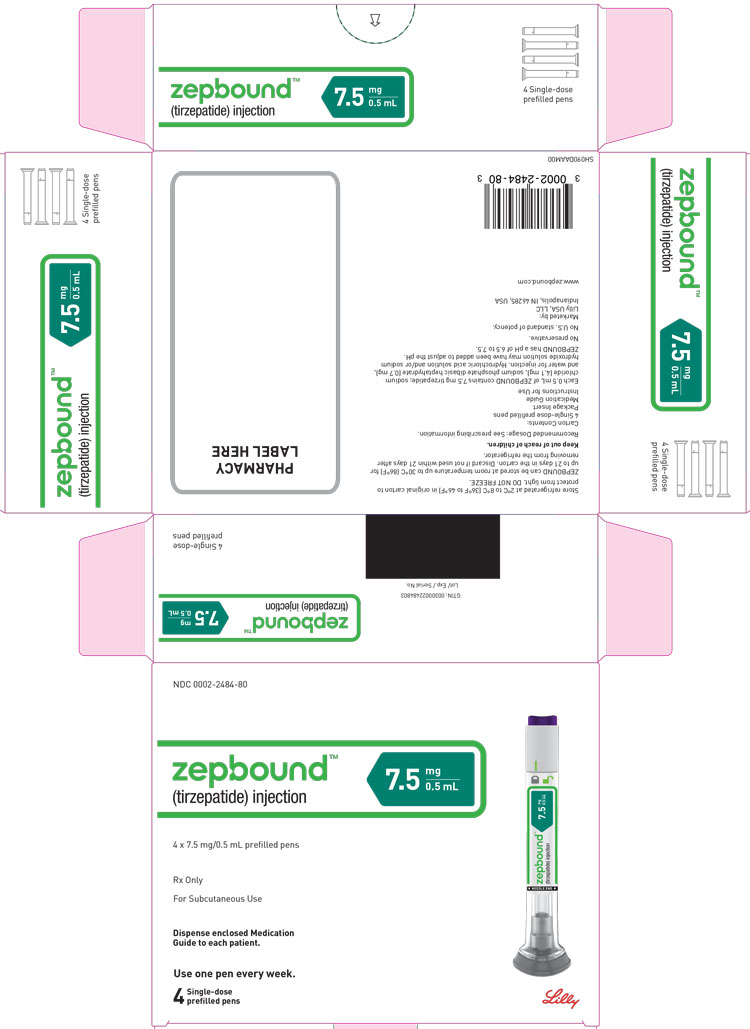
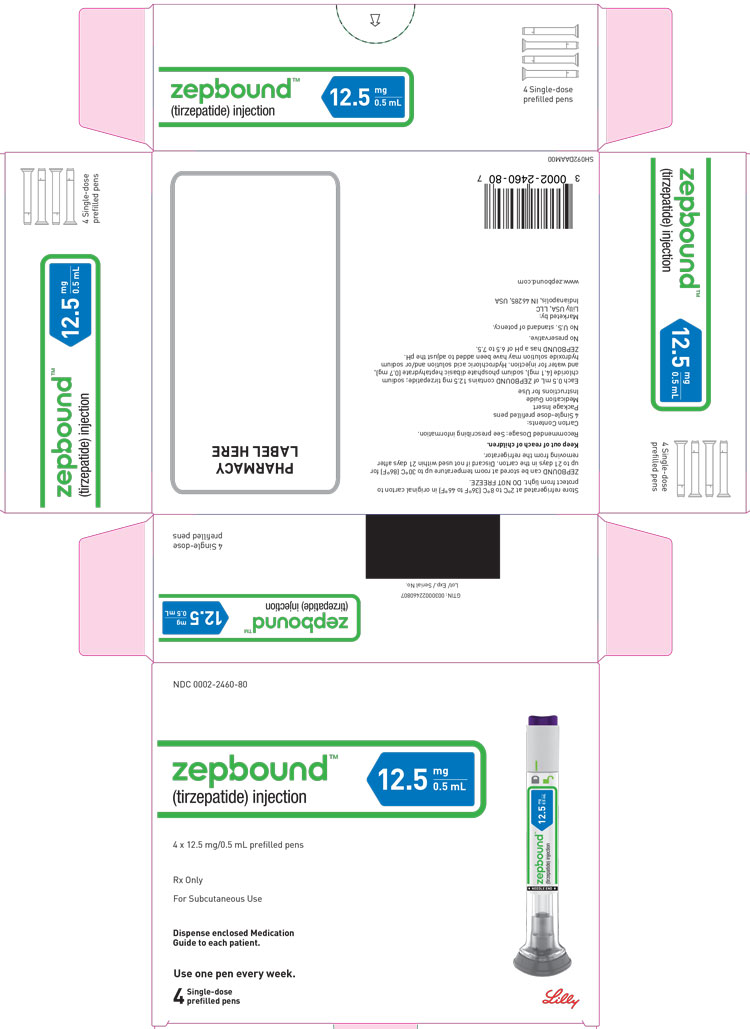
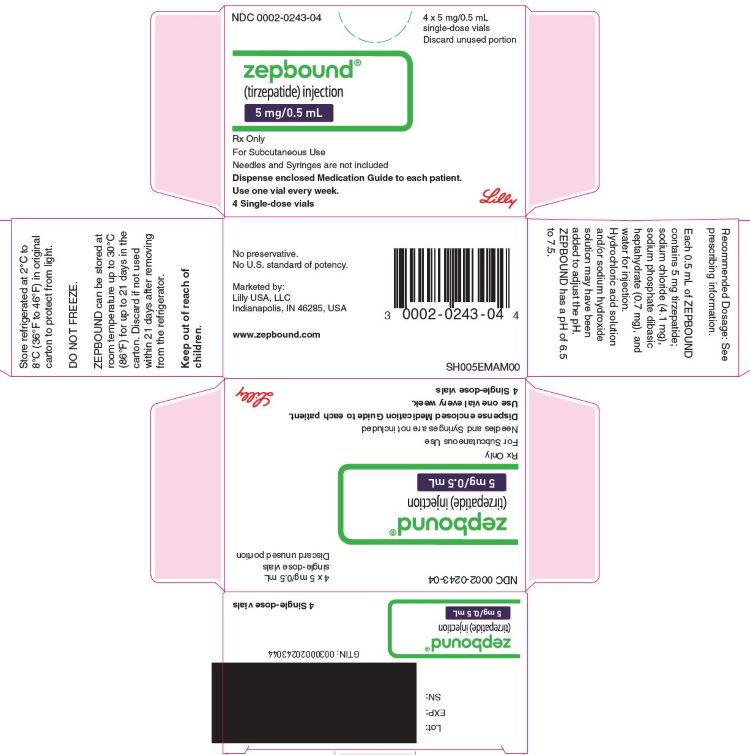
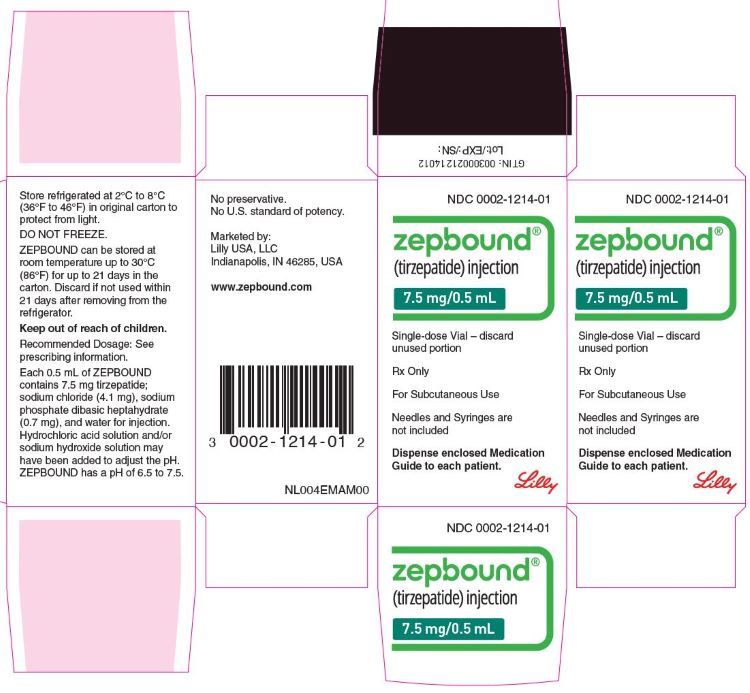
ZEPBOUND is a clear, colorless to slightly yellow solution available in cartons containing 4 pre-filled single-dose pens, 1 single-dose vial, or 4 single-dose vials as follows:
| Total Strength per Total Volume | 4 pack Pen NDC |
1 pack Vial NDC |
4 pack Vial NDC |
| 2.5 mg/0.5 mL | 0002-2506-80 | 0002-0152-01 | 0002-0152-04 |
| 5 mg/0.5 mL | 0002-2495-80 | 0002-0243-01 | 0002-0243-04 |
| 7.5 mg/0.5 mL | 0002-2484-80 | 0002-1214-01 | 0002-1214-04 |
| 10 mg/0.5 mL | 0002-2471-80 | 0002-1340-01 | 0002-1340-04 |
| 12.5 mg/0.5 mL | 0002-2460-80 | 0002-1423-01 | 0002-1423-04 |
| 15 mg/0.5 mL | 0002-2457-80 | 0002-2002-01 | 0002-2002-04 |
16.2 Storage and Handling
- Store ZEPBOUND in a refrigerator at 2°C to 8°C (36°F to 46°F).
- If needed, each single-dose pen or single-dose vial can be stored unrefrigerated at temperatures not to exceed 30°C (86°F) for up to 21 days. If ZEPBOUND is stored at room temperature, it should not be returned to the refrigerator.
- Discard if not used within 21 days after removing from the refrigerator.
- Do not freeze ZEPBOUND. Do not use ZEPBOUND if frozen.
- Store ZEPBOUND in the original carton to protect from light.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risk of Thyroid C-Cell Tumors
Inform patients that ZEPBOUND causes thyroid C-cell tumors in rats and that the human relevance of this finding has not been determined. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, persistent hoarseness, dysphagia, or dyspnea) to their healthcare provider [see Boxed Warning and Warnings and Precautions (5.1)].
Severe Gastrointestinal Adverse Reactions
Inform patients of the potential risk of severe gastrointestinal adverse reactions. Instruct patients to contact their healthcare provider if they have severe or persistent gastrointestinal symptoms [see Warnings and Precautions (5.2)].
Acute Kidney Injury
Advise patients treated with ZEPBOUND of the potential risk of dehydration due to gastrointestinal adverse reactions and take precautions to avoid fluid depletion. Inform patients of the potential risk for worsening renal function and explain the associated signs and symptoms of renal impairment, as well as the possibility of dialysis as a medical intervention if renal failure occurs [see Warnings and Precautions (5.3)].
Acute Gallbladder Disease
Inform patients of the risk of acute gallbladder disease. Instruct patients to contact their healthcare provider for appropriate clinical follow-up if gallbladder disease is suspected [see Warnings and Precautions (5.4)].
Acute Pancreatitis
Inform patients of the potential risk for pancreatitis. Instruct patients to discontinue ZEPBOUND promptly and contact their healthcare provider if pancreatitis is suspected (severe abdominal pain that may radiate to the back, and which may or may not be accompanied by vomiting) [see Warnings and Precautions (5.5)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported with use of tirzepatide. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking ZEPBOUND and seek medical advice promptly if such symptoms occur [see Warnings and Precautions (5.6)].
Hypoglycemia
Inform patients of the risk of hypoglycemia and educate patients on the signs and symptoms of hypoglycemia. Advise patients on insulin or insulin secretagogue therapy that they may have an increased risk of hypoglycemia when using ZEPBOUND and to report signs and/or symptoms of hypoglycemia to their healthcare provider [see Warnings and Precautions (5.7)].
Diabetic Retinopathy Complications
Inform patients with type 2 diabetes mellitus to contact their healthcare provider if changes in vision are experienced during treatment with ZEPBOUND [see Warnings and Precautions (5.8)].
Suicidal Behavior and Ideation
Advise patients to report emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Inform patients that if they experience suicidal thoughts or behaviors, they should stop taking ZEPBOUND [see Warnings and Precautions (5.9)].
Pulmonary Aspiration During General Anesthesia or Deep Sedation
Inform patients that ZEPBOUND may cause their stomach to empty more slowly which may lead to complications with anesthesia or deep sedation during planned surgeries or procedures. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking ZEPBOUND [see Warnings and Precautions (5.10)].
Pregnancy
Advise a pregnant patient of the potential risk to a fetus. Advise patients to inform their healthcare provider if they are pregnant or intend to become pregnant during treatment with ZEPBOUND. Advise patients that there will be a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ZEPBOUND during pregnancy [see Use in Specific Populations (8.1)].
Contraception
Use of ZEPBOUND may reduce the efficacy of oral hormonal contraceptives. Advise patients using oral hormonal contraceptives to switch to a non-oral contraceptive method or add a barrier method of contraception for 4 weeks after initiation with ZEPBOUND and for 4 weeks after each dose escalation [see Drug Interactions (7.2), Use in Specific Populations (8.3), and Clinical Pharmacology (12.3)].
Administration
Instruct patients how to prepare and administer the correct dose of ZEPBOUND and assess their ability to inject subcutaneously to ensure the proper administration of ZEPBOUND. Instruct patients using the single-dose vial to use a syringe appropriate for dose administration (e.g., a 1 mL syringe capable of measuring a 0.5 mL dose) [see Dosage and Administration (2.3)].
Missed Doses
Inform patients if a dose is missed, it should be administered as soon as possible within 4 days after the missed dose. If more than 4 days have passed, the missed dose should be skipped and the next dose should be administered on the regularly scheduled day. In each case, inform patients to resume their regular once weekly dosing schedule [see Dosage and Administration (2.2)].
Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA
Copyright © 2023, 2024, Eli Lilly and Company. All rights reserved.
ZEP-0006-USPI-20241023
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: 10/2024 |
||||||||
| Medication Guide ZEPBOUND® (ZEHP-bownd) (tirzepatide) injection, for subcutaneous use |
|||||||||
|
What is the most important information I should know about ZEPBOUND?
|
|||||||||
What is ZEPBOUND?
|
|||||||||
Do not use ZEPBOUND if:
|
|||||||||
Before using ZEPBOUND, tell your healthcare provider about all of your medical conditions,
including if you:
|
|||||||||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
ZEPBOUND may affect the way some medicines work, and some medicines may affect the
way ZEPBOUND works. Before using ZEPBOUND, tell your healthcare provider if you are taking medicines to treat diabetes including an insulin or sulfonylurea which could increase your risk of low blood sugar. Talk to your healthcare provider about low blood sugar levels and how to manage them. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
|||||||||
How should I use ZEPBOUND?
|
|||||||||
| What are the possible side effects of ZEPBOUND? ZEPBOUND may cause serious side effects, including:
|
|||||||||
| The most common side effects of ZEPBOUND include: | |||||||||
|
|
|
|||||||
| Talk to your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of ZEPBOUND. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||||||||
How should I store ZEPBOUND?
|
|||||||||
| Keep ZEPBOUND and all medicines out of the reach of children. | |||||||||
| General information about the safe and effective use of ZEPBOUND. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ZEPBOUND for a condition for which it was not prescribed. Do not give ZEPBOUND to other people, even if they have the same condition you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZEPBOUND that is written for health professionals. |
|||||||||
| What are the ingredients in ZEPBOUND? Active ingredient: tirzepatide Inactive ingredients: sodium chloride, sodium phosphate dibasic heptahydrate, and water for injection. Hydrochloric acid solution and/or sodium hydroxide solution may have been added to adjust the pH. ZEPBOUND® is a registered trademark of Eli Lilly and Company. Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA Copyright © 2023, 2024, Eli Lilly and Company. All rights reserved. For more information, go to www.zepbound.com or call 1-800-545-5979. |
|||||||||
ZEP-0003-MG-20241018
SINGLE-DOSE PEN INSTRUCTIONS FOR USE
Important information you need to know before injecting ZEPBOUND
Read this Instructions for Use and the Medication Guide before using your ZEPBOUND pen and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Talk to your healthcare provider about how to inject ZEPBOUND the right way.
- ZEPBOUND is a single-dose prefilled pen.
- ZEPBOUND is used 1 time each week.
- Inject under the skin (subcutaneously) only.
- You or another person can inject into your stomach (abdomen) or thigh.
- Another person can inject into the back of your upper arm.
Guide to parts
Preparing to inject ZEPBOUND
| Step 3 |
Place clear base on skin, then unlock | |
 |
Place the clear base flat against your skin at the injection site. | |
 |
Unlock by turning the lock ring. | |
| After your injection, place the used pen in a sharps container. |
| See Disposing of your used pen. |
- Put your used pen in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) pens in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household
container that is:
- -
- made of a heavy-duty plastic,
- -
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- -
- upright and stable during use,
- -
- leak-resistant, and
- -
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.
Storage and handling
- Store your pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store your pen at room temperature up to 86°F (30°C) for up to 21 days. If you store the pen at room temperature, do not return the pen to the refrigerator.
- Discard the pen if not used within 21 days after removing from the refrigerator.
- Do not freeze your pen. If the pen has been frozen, throw the pen away and use a new pen.
- Store your pen in the original carton to protect your pen from light.
- The pen has glass parts. Handle it carefully. If you drop the pen on a hard surface, do not use it. Use a new pen for your injection.
- Keep your ZEPBOUND pen and all medicines out of the reach of children.
Commonly asked questions
What if I see air bubbles in my pen?
Air bubbles are normal.
What if my pen is not at room temperature?
It is not necessary to warm the pen to room temperature.
What if I unlock the pen and press the purple injection button before pulling off the gray base cap?
Do not remove the gray base cap. Throw away the pen and get a new pen.
What if there is a drop of liquid on the tip of the needle when I remove the gray base cap?
A drop of liquid on the tip of the needle is normal. Do not touch the needle.
Do I need to hold the injection button down until the injection is complete?
This is not necessary, but it may help you keep the pen steady against your skin.
I heard more than 2 clicks during my injection—2 loud clicks and 1 soft one. Did I get my complete injection?
Some people may hear a soft click right before the second loud click. That is the normal operation of the pen. Do not remove the pen from your skin until you hear the second loud click.
I am not sure if my pen worked the right way.
 |
Check to see if you have received your dose. Your dose was delivered the right way
if the gray plunger is visible. Also, see Step 4 of the instructions. If you do not see the gray plunger, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) for further instructions. Until then, store your pen safely to avoid an accidental needle stick. |
What if there is a drop of liquid or blood on my skin after my injection?
This is normal. Press a cotton ball or gauze over the injection site. Do not rub the injection site.
Other information
- If you have vision problems, do not use your pen without help from a person trained to use the ZEPBOUND pen.
Where to learn more
- If you have questions or problems with your ZEPBOUND pen, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider.
- For more information about the ZEPBOUND pen, visit our website at www.zepbound.com.
 |
Scan this code to launch www.zepbound.com |
Marketed by:
Lilly USA, LLC
Indianapolis, IN 46285, USA
ZEPBOUND is a trademark of Eli Lilly and Company.
Copyright © 2023, Eli Lilly and Company. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: November 2023
ZEP-0002-PEN-IFU-20231109
SINGLE-DOSE VIAL INSTRUCTIONS FOR USE
| INSTRUCTIONS FOR USE ZEPBOUND® [ZEHP-bownd] (tirzepatide) injection, for subcutaneous use 2.5 mg/0.5 mL single-dose vial 5 mg/0.5 mL single-dose vial 7.5 mg/0.5 mL single-dose vial 10 mg/0.5 mL single-dose vial 12.5 mg/0.5 mL single-dose vial 15 mg/0.5 mL single-dose vial |
Important information you need to know before injecting ZEPBOUND
Read this Instructions for Use before you start taking ZEPBOUND and each time you get a new vial. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your needles or syringes with other people. You may give other people a serious infection or get a serious infection from them.
Talk to your healthcare provider about how to inject ZEPBOUND the right way.
- ZEPBOUND is a single-dose vial.
- ZEPBOUND is used 1 time each week.
- Inject under the skin (subcutaneously) only.
- You or another person may inject into your stomach (abdomen) or thigh.
- Another person can inject into the back of your upper arm.
Gather supplies needed to give your injection
- 1 single-dose ZEPBOUND vial
- 1 syringe and 1 needle, supplied separately (for example, use a 1 mL syringe and needle as recommended by your healthcare provider)
- 1 alcohol swab
- gauze
- 1 sharps container for throwing away used needles and syringes. See “Disposing of used needles and syringes” at the end of these instructions.
| Guide to parts Vial |
Needle and Syringe (not included) |
 |
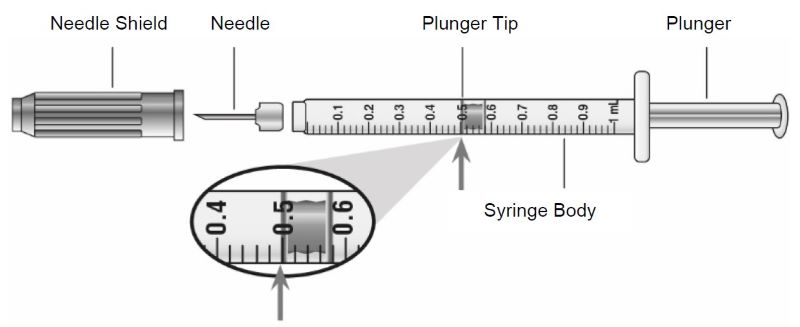 |
Note: The needle and syringe are not included. The needle and syringe recommended by your healthcare provider may look different than the needle and syringe in this Instructions for Use.
Preparing to inject ZEPBOUND
Remove the vial from the refrigerator.
Check the vial label to make sure you have the right medicine and dose, and that it has not expired.
Make sure the medicine:
|
|
Always use a new syringe and needle for each injection to prevent infections and blocked needles. Do not reuse or share your syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
Wash your hands with soap and water.
Injecting ZEPBOUND
- Inject exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you should pinch the skin before injecting.
- Change (rotate) your injection site within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not mix ZEPBOUND with any other medicine.
- Do not inject ZEPBOUND in the same injection site used for other medicines.
Disposing of used needles and syringes
- Put your used needle and syringe in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household
container that is:
- -
- made of a heavy-duty plastic,
- -
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- -
- upright and stable during use,
- -
- leak-resistant, and
- -
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community
guidelines for the right way to dispose of your sharps disposal container. There may
be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about
sharps disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal. - Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Storing ZEPBOUND
- Store all unopened vials in the refrigerator at 36°F to 46°F (2°C to 8°C).
- You may store the unopened vial at room temperature up to 86°F (30°C) for up to 21 days.
- Do not freeze. Do not use if ZEPBOUND has been frozen.
- Store the vial in the original carton to protect from light.
- Throw away all opened vials after use, even if there is medicine left in the vial.
Keep ZEPBOUND vials, syringes, needles, and all medicines out of the reach of children.
If you have any questions or problems with your ZEPBOUND, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider for help.
Marketed by:
Lilly USA, LLC
Indianapolis, IN 46285, USA
ZEPBOUND is a registered trademark of Eli Lilly and Company.
Copyright © 2024, Eli Lilly and Company. All rights reserved.
ZEP-0001-VL-IFU-20240328
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: March 2024
PACKAGE LABEL - ZepboundTM, 2.5 mg/0.5 mL, Carton, 4 Single-Dose Pens
NDC 0002-2506-80
ZepboundTM
(tirzepatide) injection
2.5 mg/0.5 mL
4 x 2.5 mg/0.5 mL prefilled pens
Rx Only
For Subcutaneous Use
Dispense enclosed Medication Guide to each patient.
Use one pen every week.
4 Single-dose prefilled pens
Lilly
PACKAGE LABEL - ZepboundTM, 5 mg/0.5 mL, Carton, 4 Single-Dose Pens
NDC 0002-2495-80
ZepboundTM
(tirzepatide) injection
5 mg/0.5 mL
4 x 5 mg/0.5 mL prefilled pens
Rx Only
For Subcutaneous Use
Dispense enclosed Medication Guide to each patient.
Use one pen every week.
4 Single-dose prefilled pens
Lilly
PACKAGE LABEL - ZepboundTM, 7.5 mg/0.5 mL, Carton, 4 Single-Dose Pens
NDC 0002-2484-80
ZepboundTM
(tirzepatide) injection
7.5 mg/0.5 mL
4 x 7.5 mg/0.5 mL prefilled pens
Rx Only
For Subcutaneous Use
Dispense enclosed Medication Guide to each patient.
Use one pen every week.
4 Single-dose prefilled pens
Lilly
PACKAGE LABEL - ZepboundTM, 10 mg/0.5 mL, Carton, 4 Single-Dose Pens
NDC 0002-2471-80
ZepboundTM
(tirzepatide) injection
10 mg/0.5 mL
4 x 10 mg/0.5 mL prefilled pens
Rx Only
For Subcutaneous Use
Dispense enclosed Medication Guide to each patient.
Use one pen every week.
4 Single-dose prefilled pens
Lilly
PACKAGE LABEL - ZepboundTM, 12.5 mg/0.5 mL, Carton, 4 Single-Dose Pens
NDC 0002-2460-80
ZepboundTM
(tirzepatide) injection
12.5 mg/0.5 mL
4 x 12.5 mg/0.5 mL prefilled pens
Rx Only
For Subcutaneous Use
Dispense enclosed Medication Guide to each patient.
Use one pen every week.
4 Single-dose prefilled pens
Lilly
PACKAGE LABEL - ZepboundTM, 15 mg/0.5 mL, Carton, 4 Single-Dose Pens
NDC 0002-2457-80
ZepboundTM
(tirzepatide) injection
15 mg/0.5 mL
4 x 15 mg/0.5 mL prefilled pens
Rx Only
For Subcutaneous Use
Dispense enclosed Medication Guide to each patient.
Use one pen every week.
4 Single-dose prefilled pens
Lilly
PACKAGE LABEL - Zepbound®, 2.5 mg/0.5 mL, Carton, 4 Single-dose Vials
NDC 0002-0152-04
Zepbound®
(tirzepatide) injection
2.5 mg/0.5 mL
4 x 2.5 mg/0.5 mL single-dose vials
Discard unused portion
Rx Only
For Subcutaneous Use
Needles and Syringes are not included
Dispense enclosed Medication Guide to each patient.
Use one vial every week.
4 Single-dose vials
Lilly
PACKAGE LABEL - Zepbound®, 5 mg/0.5 mL, Carton, 4 Single-dose Vials
NDC 0002-0243-04
Zepbound®
(tirzepatide) injection
5 mg/0.5 mL
4 x 5 mg/0.5 mL single-dose vials
Discard unused portion
Rx Only
For Subcutaneous Use
Needles and Syringes are not included
Dispense enclosed Medication Guide to each patient.
Use one vial every week.
4 Single-dose vials
Lilly
PACKAGE LABEL - Zepbound®, 7.5 mg/0.5 mL, Single-dose Vial
NDC 0002-1214-01
Zepbound®
(tirzepatide) injection
7.5 mg/0.5 mL
Single-dose Vial - discard unused portion
Rx Only
For Subcutaneous Use
Needles and Syringes are not included
Dispense enclosed Medication Guide to each patient.
Lilly
PACKAGE LABEL - Zepbound®, 10 mg/0.5 mL, Carton, Single-dose Vial
NDC 0002-1340-01
Zepbound®
(tirzepatide) injection
10 mg/0.5 mL
Single-dose Vial - discard unused portion
Rx Only
For Subcutaneous Use
Needles and Syringes are not included
Dispense enclosed Medication Guide to each patient.
Lilly