FULL PRESCRIBING INFORMATION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Evaluations and Immunizations Prior to Treatment Initiation
- Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with OMVOH [see Warnings and Precautions (5.3)].
- Obtain liver enzymes and bilirubin levels prior to initiating treatment with OMVOH [see Warnings and Precautions (5.4)].
- Complete all age-appropriate vaccinations according to current immunization guidelines [see Warnings and Precautions (5.5)].
2.2 Recommended Dosage
Induction Dosage
The recommended induction dosage of OMVOH is 300 mg administered by intravenous infusion over at least 30 minutes at Week 0, Week 4, and Week 8 [see Dosage and Administration (2.3)].
Maintenance Dosage
The recommended maintenance dosage of OMVOH is 200 mg administered by subcutaneous injection (given as two consecutive injections of 100 mg each) at Week 12, and every 4 weeks thereafter [see Dosage and Administration (2.4)].
2.3 Preparation and Administration of OMVOH for Intravenous Infusion
- OMVOH for intravenous use is intended for administration by a healthcare provider using aseptic technique. Each vial is for single use only.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be a clear to opalescent, colorless to slightly yellow to slightly brown solution, and free of visible particles. Do not use OMVOH if it is cloudy or there are visible particles.
- Using an 18 to 21 gauge needle withdraw 15 mL of OMVOH solution from the vial and transfer to an infusion bag ranging in size from 50 mL to 250 mL of 0.9% Sodium Chloride Injection or 5% Dextrose Injection. Do not mix with other drugs. Do not dilute or infuse through the same intravenous line with solutions other than 0.9% Sodium Chloride or 5% Dextrose Injection.
- Gently invert the infusion bag to mix the contents. Do not shake the prepared infusion bag.
- Connect the intravenous administration set (infusion line) to the prepared infusion bag and prime the line.
- Administer the infusion over at least 30 minutes.
- At the end of the infusion, flush the line with 0.9% Sodium Chloride Injection or
5% Dextrose Injection.
- Administer the flush at the same infusion rate as used for OMVOH administration.
- The time required to flush OMVOH solution from the infusion line is in addition to the minimum 30-minute infusion time.
Storage of Diluted Solution
- Start the infusion immediately after preparation. If not used immediately, store the diluted infusion solution in the refrigerator at 2°C to 8°C (36°F to 46°F). Use the diluted infusion solution within 48 total hours, of which not more than 5 hours are permitted at non-refrigerated temperatures not to exceed 25°C (77°F), starting from the time of vial puncture.
- Keep drug product away from direct heat or light. Do not freeze the diluted solution in the prepared infusion bag.
2.4 Preparation and Administration of OMVOH for Subcutaneous Injection
- A full maintenance dose will require 2 prefilled pens or 2 prefilled syringes.
- OMVOH is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject OMVOH after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of OMVOH according to the “Instructions for Use”, included with the packaged product.
- Before injection, remove OMVOH prefilled pen or OMVOH prefilled syringe from the refrigerator and leave at room temperature for 30 minutes. Do not shake the prefilled pens or syringes.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be a clear to opalescent, colorless to slightly yellow to slightly brown solution, and free of visible particles. Do not use OMVOH if it is cloudy, discolored, or there are visible particles.
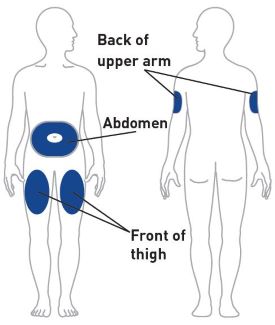
- Sites for injection include the abdomen, thigh, and back of the upper arm. Instruct patients to inject in a different location every time. For example, if the first injection was in the abdomen, administer the second injection (to complete a full dose) in another area of the abdomen, or upper arm, or thigh. Administration of OMVOH in the back of upper arm may only be performed by another person.
- Do not inject into areas where the skin is tender, bruised, erythematous, or indurated.
- OMVOH does not contain preservatives; therefore, discard any unused product. Do not reuse.
- If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing every 4 weeks.
3 DOSAGE FORMS AND STRENGTHS
OMVOH is a clear to opalescent, colorless to slightly yellow to slightly brown solution available as:
- Intravenous Infusion:
Injection: 300 mg/15 mL (20 mg/mL) solution in a single-dose vial - Subcutaneous Use:
Injection: 100 mg/mL solution in a single-dose prefilled pen
Injection: 100 mg/mL solution in a single-dose prefilled syringe
4 CONTRAINDICATIONS
OMVOH is contraindicated in patients with a history of serious hypersensitivity reaction to mirikizumab-mrkz or any of the excipients [see Warnings and Precautions (5.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis during intravenous infusion, have been reported with OMVOH administration. Infusion-related hypersensitivity reactions, including mucocutaneous erythema and pruritis, were reported during induction [see Adverse Reactions (6.1)]. If a severe hypersensitivity reaction occurs, discontinue OMVOH immediately and initiate appropriate treatment.
5.2 Infections
OMVOH may increase the risk of infection [see Adverse Reactions (6.1)].
Do not initiate treatment with OMVOH in patients with a clinically important active infection until the infection resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing OMVOH. Instruct patients to seek medical advice if signs or symptoms of clinically important acute or chronic infection occur. If a serious infection develops or an infection is not responding to standard therapy, monitor the patient closely and do not administer OMVOH until the infection resolves.
5.3 Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with OMVOH.
Do not administer OMVOH to patients with active TB infection. Initiate treatment of latent TB prior to administering OMVOH. Consider anti-TB therapy prior to initiation of OMVOH in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after OMVOH treatment.
In clinical trials, subjects were excluded if they had evidence of active TB, a past history of active TB, or were diagnosed with latent TB at screening.
5.4 Hepatotoxicity
A case of drug-induced liver injury (alanine aminotransferase [ALT] 18x the upper limit of normal (ULN), aspartate aminotransferase [AST] 10x ULN, and total bilirubin 2.4x ULN) in conjunction with pruritis was reported in a clinical trial subject following a longer than recommended induction regimen. OMVOH was discontinued. Liver test abnormalities eventually returned to baseline.
Evaluate liver enzymes and bilirubin at baseline and for at least 24 weeks of treatment. Monitor thereafter according to routine patient management.
Consider other treatment options in patients with evidence of liver cirrhosis. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. Instruct patients to seek immediate medical attention if they experience symptoms suggestive of hepatic dysfunction.
5.5 Immunizations
Avoid use of live vaccines in patients treated with OMVOH. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Prior to initiating therapy with OMVOH, complete all age-appropriate vaccinations according to current immunization guidelines. No data are available on the response to live or non-live vaccines in patients treated with OMVOH.
6 ADVERSE REACTIONS
The following topics are also discussed in detail in the Warnings and Precautions section:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Infections [see Warnings and Precautions (5.2)]
- Tuberculosis [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
OMVOH was studied up to 12 weeks in subjects with moderately to severely active ulcerative colitis in a randomized, double-blind, placebo-controlled induction study (UC-1). In subjects who responded to induction therapy in UC-1, long term safety up to 52 weeks was evaluated in a randomized, double-blind, placebo-controlled maintenance study (UC-2) and a long-term extension study [see Clinical Studies (14)].
In the induction study (UC-1), 1279 subjects were enrolled of whom 958 received OMVOH 300 mg administered as an intravenous infusion at Weeks 0, 4, and 8. In the maintenance study (UC-2), 581 subjects were enrolled of whom 389 received OMVOH 200 mg administered as a subcutaneous injection every 4 weeks.
Table 1 summarizes the adverse reactions reported in at least 2% of subjects and at a higher frequency than placebo during UC-1.
|
a Reported in at least 2% of subjects and at a higher frequency than placebo. |
||
|
b OMVOH 300 mg as an intravenous infusion at Weeks 0, 4, and 8. |
||
|
c Upper respiratory tract infections includes related terms (e.g., COVID-19, nasopharyngitis, pharyngitis, rhinitis, sinusitis, and upper respiratory tract infection). |
||
| Adverse Reactions | OMVOH 300 mg Intravenous Infusionb N=958 n (%) |
Placebo N=321 n (%) |
| Upper respiratory tract infectionsc | 72 (8%) | 20 (6%) |
| Arthralgia | 20 (2%) | 4 (1%) |
In the induction study (UC-1), infusion-related hypersensitivity reactions were reported by 4 (0.4%) subjects treated with OMVOH and 1 (0.3%) subject treated with placebo.
Table 2 summarizes the adverse reactions reported in at least 2% of subjects and at a higher frequency than placebo during the 40-week controlled period of UC-2.
|
a Reported in at least 2% of subjects and at a higher frequency than placebo |
||
|
b OMVOH 200 mg as a subcutaneous injection at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks. |
||
|
c Upper respiratory tract infections includes related terms (e.g., COVID-19, nasopharyngitis, pharyngitis, rhinitis, sinusitis, and upper respiratory tract infection). |
||
|
d Injection site reactions includes related terms (e.g., erythema, hypersensitivity, pain, reaction, and urticaria at the injection site). |
||
|
e Rash is composed of several similar terms. |
||
|
f Herpes viral infection includes related terms (e.g., herpes zoster, herpes simplex, and oral herpes). |
||
| Adverse Reactions | OMVOH 200 mg Subcutaneous Injectionb N=389 n (%) |
Placebo N=192 n (%) |
| Upper respiratory tract infectionsc | 53 (14%) | 23 (12%) |
| Injection site reactionsd | 34 (9%) | 8 (4%) |
| Arthralgia | 26 (7%) | 8 (4%) |
| Rashe | 16 (4%) | 2 (1%) |
| Headache | 16 (4%) | 2 (1%) |
| Herpes viral infectionf | 9 (2%) | 1 (1%) |
Infections
In UC-1 through Week 12, infections were reported by 145 (15%) subjects treated with OMVOH 300 mg and 45 (14%) subjects treated with placebo. Serious infections were reported by less than 1% in both groups. Serious infections in the OMVOH group included intestinal sepsis, listeria sepsis, and pneumonia.
In the maintenance study (UC-2) through Week 40 (a total of 52 weeks of treatment), infections were reported by 93 (24%) subjects treated with OMVOH 200 mg and 44 (23%) subjects treated with placebo. A case of COVID-19 pneumonia was reported as a serious infection in the OMVOH group.
Hepatic Enzyme Elevations
In UC-1 through Week 12, alanine aminotransferase (ALT) ≥5X ULN was reported by 1 (0.1%) subject treated with OMVOH 300 mg and 1 (0.3%) subject treated with placebo. Aspartate aminotransferase (AST) ≥5X ULN was reported by 2 (0.2%) subjects treated with OMVOH 300 mg and no subject treated with placebo. These elevations have been noted with and without concomitant elevations in total bilirubin.
In UC-2 through Week 40 (a total of 52 weeks of treatment), 3 (0.8%) subjects treated with OMVOH 200 mg reported ALT ≥5X ULN and 3 (0.8%) subjects reported AST ≥5X ULN; with or without concomitant elevations in total bilirubin. No subjects treated with placebo experienced similar elevations [see Warnings and Precautions (5.4)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There will be a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to OMVOH during pregnancy. Pregnant women exposed to OMVOH and healthcare providers are encouraged to call Eli Lilly and Company at 1-800-Lilly-Rx (1-800-545-5979).
Risk Summary
Available data from case reports of mirikizumab-mrkz use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Although there are no data on mirikizumab-mrkz, monoclonal antibodies can be actively transported across the placenta, and mirikizumab-mrkz may cause immunosuppression in the in utero-exposed infant. An enhanced pre- and post-natal development study conducted in pregnant monkeys at a dose 69 times the maximum recommended human dose (MRHD) revealed no adverse developmental effects to the developing fetus, or harm to infant monkeys from birth through 6 months of age. There are risks of adverse pregnancy outcomes associated with increased disease activity in women with inflammatory bowel disease (see Clinical Considerations).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and Embryo/Fetal Risk
Published data suggest that the risk of adverse pregnancy outcomes in women with inflammatory bowel disease (IBD) is associated with increased disease activity. Adverse pregnancy outcomes include preterm delivery (before 37 weeks gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
Fetal/Neonatal Adverse Reactions
Transport of endogenous IgG antibodies across the placenta increases as pregnancy progresses, and peaks during the third trimester. Because mirikizumab-mrkz may interfere with immune response to infections, risks and benefits should be considered prior to administering live vaccines to infants exposed to OMVOH in utero. There are no data regarding infant serum levels of mirikizumab-mrkz at birth and the duration of persistence of mirikizumab-mrkz in infant serum after birth. Although a specific timeframe to delay live virus immunizations in infants exposed in utero is unknown, a minimum of 2 months after birth should be considered because of the half-life of the product.
Data
Animal Data
An enhanced pre- and postnatal development study was conducted in cynomolgus monkeys administered mirikizumab-mrkz by intravenous injection during organogenesis to parturition at a dose of 300 mg/kg twice weekly (69 times the MRHD based on exposure comparisons). Mirikizumab-mrkz crossed the placenta in monkeys. No maternal toxicity was noted in this study. No mirikizumab-mrkz-related effects on morphological, functional or immunological development were observed in infant monkeys from birth through 6 months of age. However, incidences of embryo/fetal loss were higher in the treated groups compared to control (6.7% [1 of 15] in controls vs 26.7% [4 of 15] at 300 mg/kg (69 times the MRHD, based on exposure comparisons) but were within the range of historical control data. Following delivery, most adult female cynomolgus monkeys and all infants from the mirikizumab-mrkz-treated group had measurable serum concentrations up to 28 days postpartum. In the infant monkeys, mean serum concentrations were approximately 4.8 times the respective mean maternal concentrations.
8.2 Lactation
Risk Summary
There are no data on the presence of mirikizumab-mrkz in human milk, the effects on the breastfed infant, or the effects on milk production. Endogenous maternal IgG and monoclonal antibodies are transferred in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to mirikizumab-mrkz are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OMVOH and any potential adverse effects on the breastfed infant from OMVOH or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of OMVOH have not been established in pediatric patients.
8.5 Geriatric Use
Of the 795 OMVOH-treated subjects in the two clinical trials, 64 subjects (8%) were 65 years of age and older, while 10 subjects (1%) were 75 years of age and older. These clinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger adult subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects. No clinically meaningful differences in the pharmacokinetics of mirikizumab-mrkz were observed in subjects 65 years of age and older compared to younger adult subjects [see Clinical Pharmacology (12.3)].
11 DESCRIPTION
Mirikizumab-mrkz is a humanized immunoglobulin G4 (IgG4) variant monoclonal antibody that is directed against the p19 subunit of IL-23 and does not bind IL-12. Mirikizumab-mrkz is produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology and it is composed of two identical light chain polypeptides and two identical heavy chain polypeptides with an overall molecular weight of approximately 147 kDa.
OMVOH for Intravenous Infusion
OMVOH (mirikizumab-mrkz) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow to slightly brown solution in a single-dose vial for intravenous infusion after dilution. Each mL contains 20 mg of mirikizumab-mrkz, anhydrous citric acid (0.4 mg), polysorbate 80 (0.5 mg), sodium chloride (8.8 mg), sodium citrate (2.1 mg), and Water for Injection. The OMVOH solution has a pH range of 5.0 - 6.0.
OMVOH for Subcutaneous Injection
OMVOH (mirikizumab-mrkz) injection is a sterile, preservative free, clear to opalescent, colorless to slightly yellow to slightly brown solution for subcutaneous use available as 100 mg of mirikizumab-mrkz in a 1 mL single-dose prefilled pen or single-dose prefilled syringe. The prefilled pen and prefilled syringe each contain a 1 mL glass syringe with a fixed 27-gauge ½ inch needle. The OMVOH 100 mg prefilled pen and prefilled syringe are manufactured to deliver 100 mg of mirikizumab-mrkz. Each mL is composed of mirikizumab-mrkz (100 mg), anhydrous citric acid (0.4 mg), polysorbate 80 (0.3 mg), sodium chloride (8.8 mg), sodium citrate (2.1 mg), and Water for Injection. The OMVOH solution has a pH range of 5.0 to 6.0.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mirikizumab-mrkz is a humanized IgG4 monoclonal antibody that selectively binds to the p19 subunit of human IL-23 cytokine and inhibits its interaction with the IL-23 receptor.
IL-23 is involved in mucosal inflammation and affects the differentiation, expansion, and survival of T cell subsets, and innate immune cell subsets, which represent sources of pro-inflammatory cytokines. Research in animal models has shown that pharmacologic inhibition of IL-23p19 can ameliorate intestinal inflammation.
Mirikizumab-mrkz inhibits the release of pro-inflammatory cytokines and chemokines.
12.2 Pharmacodynamics
In both study UC-1 (induction) and study UC-2 (maintenance), a positive relationship was observed between mirikizumab-mrkz average concentration and rates of clinical remission and clinical response [see Clinical Studies (14)].
12.3 Pharmacokinetics
Mirikizumab-mrkz exhibited linear pharmacokinetics with dose-proportional increase in exposure over a dose range of 60 to 2400 mg given as an intravenous injection or over a dose range of 200 to 400 mg given as a subcutaneous injection, in healthy volunteers. There was no apparent accumulation of mirikizumab-mrkz concentrations in serum over time when administered as a subcutaneous injection every 4 weeks to subjects with ulcerative colitis. The estimated exposure parameters of mirikizumab-mrkz at steady state are summarized in Table 3.
|
a OMVOH 300 mg as an intravenous infusion over at least 30 minutes at Weeks 0, 4, and 8. |
||
|
b OMVOH 200 mg as a subcutaneous injection at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks. |
||
|
c AUCtau, ss = area under the concentration-versus-time curve over one dosing interval at steady state; Cmax, ss = maximum concentration at steady state; Ctrough, ss = concentration at the end of the dosing interval at steady state; CV = geometric coefficient of variation. |
||
| OMVOH 300 mg Intravenous Infusiona Geometric mean (CV%) |
OMVOH 200 mg Subcutaneous Injectionb Geometric mean (CV%) |
|
| Cmax, ss (µg/mL)c | 99.7 (22.7%) | 10.1 (52.1%) |
| AUCtau, ss (µg*day/mL)c | 538 (34.4%) | 160 (57.6%) |
| Ctrough, ss (µg/mL)c | 2.75 (101%) | 1.70 (83.3%) |
Absorption
Following subcutaneous dosing of OMVOH, median (range) Tmax was 5 (3.08 to 6.75) days post dose and geometric mean (CV%) absolute bioavailability was 44% (34%).
Injection site location (abdomen, upper arm, or thigh) did not significantly influence bioavailability of mirikizumab-mrkz following subcutaneous injection.
Distribution
The geometric mean (CV%) total volume of distribution in subjects with ulcerative colitis was 4.83 L (21%).
Metabolism/Elimination
Mirikizumab-mrkz is a humanized IgG4 monoclonal antibody and is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Geometric mean (CV%) clearance was 0.0229 L/hours (34%) and the geometric mean (CV%) elimination half-life was 9.3 days (40%) in subjects with ulcerative colitis. Clearance is independent of dose.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of mirikizumab-mrkz based on age (18 to 79 years), sex, race (White or Asian), or mild and moderate renal impairment (i.e., estimated creatinine clearance by Cockcroft-Gault equation: 30 to 89 mL/min).
Body Weight
Following intravenous administration of 300 mg, the recommended induction dose, in subjects with ulcerative colitis weighing 90 kg or greater, the estimated geometric mean mirikizumab-mrkz average concentration (Cavg) was 20% lower compared with subjects weighing less than 90 kg. Following subcutaneous administration of 200 mg, the recommended maintenance dose, in subjects with ulcerative colitis weighing 90 kg or greater, the estimated geometric mean Cavg was 38% lower compared with subjects weighing less than 90 kg. In Study UC-2 (maintenance), the rate of clinical remission and clinical response did not differ significantly between subjects weighing 90 kg or greater and subjects weighing less than 90 kg.
Drug Interaction Studies
Population pharmacokinetic analyses indicated that the clearance of OMVOH was not impacted by concomitant administration of aminosalicylates, corticosteroids, or oral immunomodulators (6-MP, AZA, MTX, tioguanine) in subjects with ulcerative colitis.
No drug-drug interaction studies were conducted in subjects with ulcerative colitis at the recommended dosage. Based on a clinical drug-drug interaction study conducted in subjects with another condition, multiple subcutaneous doses of 250 mg every 4 weeks of mirikizumab-mrkz (a dosage 1.25-times higher than the recommended maintenance dosage) did not result in changes in the exposure of midazolam (CYP3A substrate), warfarin (CYP2C9 substrate), dextromethorphan (CYP2D6 substrate), omeprazole (CYP2C19 substrate), or caffeine (CYP1A2 substrate).
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of mirikizumab-mrkz or of other mirikizumab products.
During the 52-week treatment period in studies UC-1 and UC-2, 23% (88/378) of OMVOH-treated subjects at the recommended dosage and evaluable for assessment, developed anti-mirikizumab-mrkz antibodies (referred to as anti-drug antibodies (ADA)). Of those who developed ADA, 33/88 (38%) developed titers ≥1:160. Of these 33 OMVOH-treated subjects, 10 had reduced serum trough concentrations of mirikizumab-mrkz compared to subjects who did not develop anti-mirikizumab-mrkz antibodies, and 5 of these 10 subjects did not achieve clinical response at Week 52. There is insufficient data to assess whether the observed ADA-associated pharmacokinetic changes reduced effectiveness. There is no identified clinically significant effect of ADA on the safety of OMVOH over the treatment duration of 52-weeks.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of mirikizumab-mrkz.
No organ weight or histopathology effects were observed in the male or female reproductive tract in sexually mature cynomolgus monkeys that received subcutaneous mirikizumab-mrkz once weekly for 26 weeks, at a dose of 100 mg/kg (at least 7 times the MRHD of mirikizumab-mrkz, based on exposure comparisons).
14 CLINICAL STUDIES
The safety and efficacy of OMVOH was evaluated in two randomized, double-blind, placebo-controlled clinical studies, one induction study [UC-1 (NCT03518086)] and one maintenance study [UC-2 (NCT03524092)], in adult subjects with moderately to severely active ulcerative colitis who had inadequate response, loss of response, or failed to tolerate any of the following: corticosteroids, 6-mercaptopurine, azathioprine, biologic therapy (TNF blocker, vedolizumab), or tofacitinib. The 12-week intravenous induction study (UC-1) was followed by the 40-week subcutaneous randomized withdrawal maintenance study (UC-2).
Study UC-1
In UC-1, efficacy was evaluated in 1062 subjects who were randomized 3:1 at Week 0 to receive 300 mg OMVOH or placebo by intravenous infusion at Week 0, Week 4, and Week 8. Subjects had a mean age of 43 years (range 18 to 79 years); 40% were female; and 71% identified as White, 25% as Asian, 1% as American Indian or Alaska Native, 1% as Black or African American, and <2% as another racial group or did not report their racial group. Subjects were permitted to use stable doses of aminosalicylates, immunomodulators (6-mercaptopurine, azathioprine, methotrexate), and oral corticosteroids (prednisone ≤20 mg/day or equivalent, extended-release budesonide 9 mg/day, beclomethasone dipropionate 5 mg/day). At baseline, 41% of subjects were receiving oral corticosteroids, 24% were receiving immunomodulators, and 75% were receiving aminosalicylates.
At baseline, 57% were biologic and Janus Kinase inhibitor (JAKi) naive, 41% had failed at least one biologic, 3% had failed a JAKi, and 2% had previously received but had not failed a biologic or JAKi.
Disease activity was assessed based on the modified Mayo score (mMS), which ranges from 0 to 9 and has three subscores that are each scored from 0 (normal) to 3 (most severe): stool frequency, rectal bleeding, and findings on centrally read endoscopy subscore. At baseline, subjects had a mMS of 5 to 9, including a centrally read endoscopy subscore of 2 or 3. An endoscopy subscore of 2 was defined by marked erythema, absent vascular pattern, friability, and erosions; and a subscore of 3 was defined by spontaneous bleeding and ulceration. Subjects had a median mMS of 7, and 58% had severely active disease (mMS of 7 to 9).
The primary endpoint was clinical remission at Week 12. The secondary endpoints were clinical response, endoscopic improvement, and histologic-endoscopic mucosal improvement (see Table 4).
|
JAKi = Janus Kinase inhibitor |
|||
|
a OMVOH 300 mg as an intravenous infusion at Week 0, Week 4, and Week 8. |
|||
|
b Adjusted treatment difference based on Cochran-Mantel-Haenszel method adjusted for randomization stratification factors. |
|||
|
c Clinical remission based on mMS is defined as: stool frequency subscore = 0 or 1, rectal bleeding subscore = 0, and centrally read endoscopy subscore = 0 or 1 (excluding friability). |
|||
|
d Tested at an alpha level of 0.00125, with a p-value <0.001. |
|||
|
e Prior biologic or JAKi failure includes loss of response, inadequate response, or intolerance to one or more biologic therapy (TNF blocker or vedolizumab), or tofacitinib. |
|||
|
f Clinical response is defined as a decrease in the mMS of ≥2 points with ≥30% decrease from baseline, and either a decrease of ≥1 point in the rectal bleeding subscore from baseline or a rectal bleeding subscore of 0 or 1. |
|||
|
g Endoscopic improvement is defined as a centrally read endoscopy subscore of 0 or 1 (excluding friability). |
|||
|
h Histologic-endoscopic mucosal improvement is defined as achieving both endoscopic improvement (centrally read endoscopy subscore of 0 or 1, excluding friability) and histologic improvement (neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue based on the Geboes scoring system). |
|||
| Endpoint | Placebo | OMVOH 300 mg Intravenous Infusiona |
Treatment Differenceb (95% CI) |
| Clinical remissionc | |||
| Total Population | N = 267 15% |
N = 795 24% |
10%d (5, 15) |
| Biologic and JAKi naive | N = 155 18% |
N = 450 31% |
|
| Prior biologic or JAKi failuree | N = 107 8% |
N = 331 15% |
|
| Clinical responsef | |||
| Total Population | N = 267 43% |
N = 795 65% |
22%d (15, 28) |
| Biologic and JAKi naive | N = 155 52% |
N = 450 71% |
|
| Prior biologic or JAKi failured, e | N = 107 31% |
N = 331 56% |
|
| Endoscopic improvementg | |||
| Total Population | N = 267 21% |
N = 795 34% |
14%d (8, 20) |
| Biologic and JAKi naive | N = 155 28% |
N = 450 44% |
|
| Prior biologic or JAKi failuree | N = 107 10% |
N = 331 22% |
|
| Histologic-endoscopic mucosal improvementh | |||
| Total Population | N = 267 14% |
N = 795 25% |
11%d (6, 16) |
| Biologic and JAKi naive | N = 155 19% |
N = 450 34% |
|
| Prior biologic or JAKi failuree | N = 107 7% |
N = 331 13% |
|
Study UC-1 was not designed to evaluate the relationship of histologic-endoscopic mucosal improvement at Week 12 to disease progression and long-term outcomes.
Rectal Bleeding and Stool Frequency Subscores
Decreases in rectal bleeding and stool frequency subscores were observed as early as Week 3 in subjects treated with OMVOH compared to subjects on placebo.
Study UC-2
The maintenance study (UC-2) evaluated 506 subjects who achieved clinical response at Week 12 in Study UC-1. These subjects were randomized 2:1 to receive 200 mg OMVOH or placebo subcutaneously every 4 weeks for 40 weeks in UC-2, for a total of 52 weeks of treatment. Subjects who were on concomitant ulcerative colitis therapies during UC-1 were required to continue on stable doses of oral aminosalicylates and immunomodulators (6-mercaptopurine, azathioprine, methotrexate). Corticosteroid tapering was required for subjects who were receiving corticosteroids at baseline and achieved clinical response in UC-1.
The primary endpoint was clinical remission at Week 40. The secondary endpoints were endoscopic improvement, maintenance of clinical remission in subjects who achieved clinical remission at Week 12, corticosteroid-free clinical remission, and histologic-endoscopic mucosal improvement (see Table 5).
|
JAKi = Janus Kinase inhibitor |
|||
|
a The placebo arm includes subjects treated with OMVOH during the induction study (UC-1) and were randomized to receive placebo through Week 40. |
|||
|
b OMVOH 200 mg as a subcutaneous injection at Week 12 and every 4 weeks thereafter for up to an additional 40 weeks. |
|||
|
c Adjusted treatment difference (95% CI) based on Cochran-Mantel-Haenszel method adjusted for randomization stratification factors. |
|||
|
d Among subjects who achieved clinical response at Week 12 in UC-1 with OMVOH induction treatment. |
|||
|
e Clinical remission based on mMS is defined as: stool frequency subscore = 0 or 1, rectal bleeding subscore = 0, and centrally read endoscopy subscore = 0 or 1 (excluding friability). |
|||
|
f p<0.001. |
|||
|
g Prior biologic or JAKi failure includes loss of response, inadequate response, or intolerance to one or more biologic therapy (TNF blocker or vedolizumab), or tofacitinib. |
|||
|
h Endoscopic improvement is defined as a centrally read endoscopy subscore of 0 or 1 (excluding friability). |
|||
|
i Among subjects who achieved clinical remission at Week 12 in UC-1 with OMVOH induction treatment. |
|||
|
j p<0.01. |
|||
|
k Corticosteroid-free clinical remission is defined as clinical remission at Week 40 and no corticosteroid use for ≥12 weeks prior to Week 40 assessment. |
|||
|
l Histologic-endoscopic mucosal improvement is defined as achieving both endoscopic improvement (centrally read endoscopy subscore of 0 or 1, excluding friability) and histologic improvement (no neutrophils in crypts or lamina propria, no crypt destruction, and no erosions, ulcerations, or granulation tissue based on the Geboes scoring system). |
|||
| Endpoint | Placeboa | OMVOH 200 mg Subcutaneous Injectionb |
Treatment Differencec (95% CI) |
| Clinical remissiond, e | |||
| Total Population | N = 169 27% |
N = 337 51% |
22%f (14, 31) |
| Biologic and JAKi naive | N = 109 33% |
N = 208 53% |
|
| Prior biologic or JAKi failureg | N = 59 15% |
N = 121 45% |
|
| Endoscopic improvementd, h | |||
| Total Population | N = 169 30% |
N = 337 58% |
27%f (19, 36) |
| Biologic and JAKi naive | N = 109 35% |
N = 208 62% |
|
| Prior biologic or JAKi failureg | N = 59 20% |
N = 121 50% |
|
| Maintenance of clinical remission in patients who achieved clinical remission at Week 12i | |||
| Total Population | N = 62 40% |
N = 128 66% |
23%j (8, 38) |
| Biologic and JAKi naive | N = 48 48% |
N = 91 66% |
|
| Prior biologic or JAKi failureg | N = 14 14% |
N = 34 65% |
|
| Corticosteroid-free clinical remissiond, k | |||
| Total Population | N = 169 27% |
N = 337 50% |
22%f (13, 30) |
| Biologic and JAKi naive | N = 109 33% |
N = 208 52% |
|
| Prior biologic or JAKi failureg | N = 59 15% |
N = 121 45% |
|
| Histologic-endoscopic mucosal improvementd, l | |||
| Total Population | N = 169 22% |
N = 337 43% |
19%f (11, 27) |
| Biologic and JAKi naive | N = 109 27% |
N = 208 47% |
|
| Prior biologic or JAKi failureg | N = 59 14% |
N = 121 36% |
|
Study UC-2 was not designed to evaluate the relationship of histologic-endoscopic mucosal improvement at Week 40 to disease progression and long-term outcomes.
Bowel Urgency
Bowel urgency was assessed during UC-1 and UC-2 with an Urgency Numeric Rating Scale (NRS) of 0 to 10. A greater proportion of subjects with a baseline Urgency NRS weekly average score ≥3 treated with OMVOH compared to placebo reported an Urgency NRS weekly average score of 0 or 1 (39% versus 23%) at Week 40. Urgency NRS weekly average scores of 0 to 1 were also observed in a greater proportion of subjects treated with OMVOH compared to placebo at Week 12.
Endoscopic Assessment
Normalization of the endoscopic appearance of the mucosa (endoscopic remission) was defined as a Mayo endoscopic subscore of 0. At Week 40 in UC-2, endoscopic remission was observed in a greater proportion of subjects treated with OMVOH compared to placebo (22% versus 14%).
16 HOW SUPPLIED/STORAGE AND HANDLING
OMVOH (mirikizumab-mrkz) injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow to slightly brown solution for intravenous infusion or subcutaneous injection.
OMVOH is supplied as:
| Strength | Pack Size | NDC Code | |
| For Intravenous Infusion | |||
| Single-dose Vial | 300 mg/15 mL (20 mg/mL) |
Carton of 1 | 0002-7575-01 |
| For Subcutaneous Use | |||
| Single-dose Prefilled Pen | 100 mg/mL | Carton of 2 | 0002-8011-27 |
| Single-dose Prefilled Syringe | 100 mg/mL | Carton of 2 | 0002-8870-27 |
Each single-dose prefilled pen or prefilled syringe consists of a 1 mL glass syringe with a fixed 27-gauge ½ inch needle.
Storage and Handling
- Store refrigerated at 2°C to 8°C (36°F to 46°F).
- Do not freeze. Do not use OMVOH if it has been frozen.
- Do not shake.
- Keep OMVOH in the original carton to protect from light until the time of use.
- OMVOH is sterile and preservative-free. Discard any unused portion.
- If needed, the prefilled pen or prefilled syringe may be stored at room temperature up to 30°C (86°F) for up to 2 weeks in the original carton to protect from light. Once OMVOH has been stored at room temperature, do not return to the refrigerator. If these conditions are exceeded, OMVOH must be discarded.
- The vial, prefilled pen, and prefilled syringe are not made with dry natural rubber latex.
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Hypersensitivity Reactions
Advise patients to discontinue OMVOH and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.1)].
Infections
Advise patients that OMVOH may lower the ability of their immune system to fight infections and to contact their healthcare provider immediately if they develop any symptoms of infection [see Warnings and Precautions (5.2)].
Tuberculosis
Advise patients to contact their healthcare provider if they experience symptoms suggestive of TB (e.g., unexplained fever, cough, or difficulty breathing) [see Warnings and Precautions (5.3)].
Hepatotoxicity
Inform patients that OMVOH may cause liver injury. Advise patients to seek immediate medical attention if they experience symptoms suggestive of liver dysfunction (e.g., unexplained rash, nausea, vomiting, abdominal pain, fatigue, anorexia, or jaundice and/or dark urine) [see Warnings and Precautions (5.4)].
Immunizations
Advise patients that vaccination with live vaccines is not recommended during OMVOH treatment and immediately prior to or after OMVOH treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform their healthcare provider that they are taking OMVOH prior to receiving a vaccination [see Warnings and Precautions (5.5)].
Pregnancy
Advise patients who are exposed to OMVOH during pregnancy to contact Eli Lilly and Company [see Use in Specific Populations (8.1)].
Administration
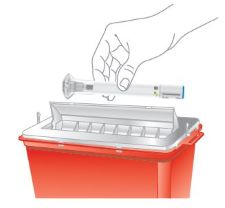
Instruct patients in preparation and administration of OMVOH, including choosing anatomical sites for subcutaneous administration, and proper subcutaneous injection technique. Instruct patients in the technique of pen or syringe disposal [see Instructions for Use].
Instruct patients or caregivers to administer two 100 mg prefilled pens or two 100 mg prefilled syringes to achieve the full 200 mg dose of OMVOH.
Eli Lilly and Company, Indianapolis, IN 46285, USA
US License No. 1891
Copyright © 2023, 2024, Eli Lilly and Company. All rights reserved.
OMV-0002-USPI-20240429
OMV-0002-MG-20240429
PREFILLED PEN INSTRUCTIONS FOR USE
| INSTRUCTIONS FOR USE |
| OMVOHTM (ahm-VOH) |
| (mirikizumab-mrkz) |
| injection, for subcutaneous use |
| 100 mg/mL prefilled pen |
 |
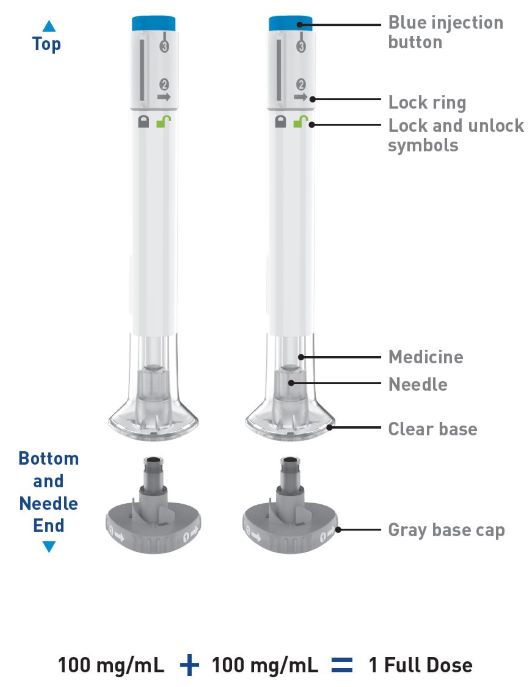
| This Instructions for Use contains information on how to inject OMVOH. |
| Before you use the OMVOH prefilled pens (Pens), read and carefully follow all the step-by-step instructions. Two injections are required for a full dose. |
| Important information you need to know before injecting OMVOH: |
|
|
|
|
|
|
|
|
|
|
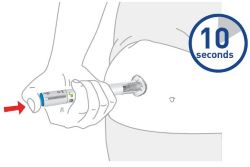
| 2 injections are required for a full dose. Inject one Pen immediately followed by the other Pen. |
| Preparing to inject OMVOH | |
| Take the Pens from the refrigerator | Take 2 OMVOH Pens from the refrigerator. |
| Leave the gray base caps on until you are ready to inject. | |
| Leave the Pens at room temperature for 30 minutes before injecting. | |
| Do not microwave the Pens, or run hot water over them, or leave them in direct sunlight. | |
| Do not shake the Pens. | |
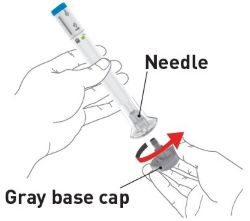
| Gather supplies | Supplies:
|
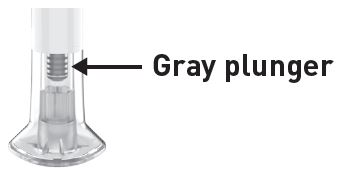
| Inspect the Pens and the medicine Expiration date  |
Make sure you have the right medicine. The medicine inside should be clear. It may
be colorless to slightly yellow to slightly brown. Do not use the Pens and throw away (dispose of) as directed by your healthcare provider or pharmacist if:
|
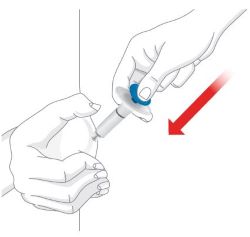
| Prepare for injection | Wash your hands with soap and water before you inject OMVOH. |
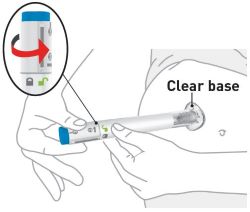
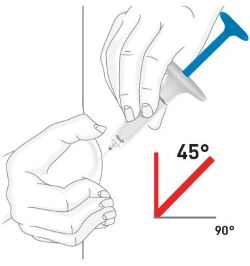
| Your healthcare provider can help you choose the injection site that is best for you. | |
 |
|
|
|
|
|
|
|
|
|
| Clean the injection sites with an alcohol wipe. Let the injection sites dry before you inject the medicine. | |
|
|
|
|
|
|
|
|
| Read the Medication Guide for OMVOH inside this box to learn more about your medicine. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891
OMVOHTM is a trademark of Eli Lilly and Company.
Copyright © 2023, 2024, Eli Lilly and Company. All rights reserved.
Revised: May 17, 2024
The OMVOH Pen meets the current dose accuracy and functional requirements of ISO 11608-1 and 11608-5.
OMV-0003-IFU-PEN-20240517
PREFILLED SYRINGE INSTRUCTIONS FOR USE
| INSTRUCTIONS FOR USE |
| OMVOHTM (ahm-VOH) |
| (mirikizumab-mrkz) |
| injection, for subcutaneous use |
| 100 mg/mL prefilled syringe |
| This Instructions for Use contains information on how to inject OMVOH. |
 |
| Before you use the OMVOH prefilled syringes, read and carefully follow all the step-by-step instructions. Two injections are required for a full dose. |
| Important information you need to know before injecting OMVOH: |
|
|
|
|
|
|
|
|
|
|
| INSTRUCTIONS FOR USE |
| Before you use OMVOH prefilled syringes, read and carefully follow all the step-by-step instructions. |
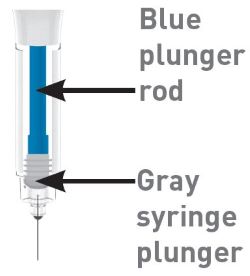
| Parts of the OMVOH prefilled syringe |
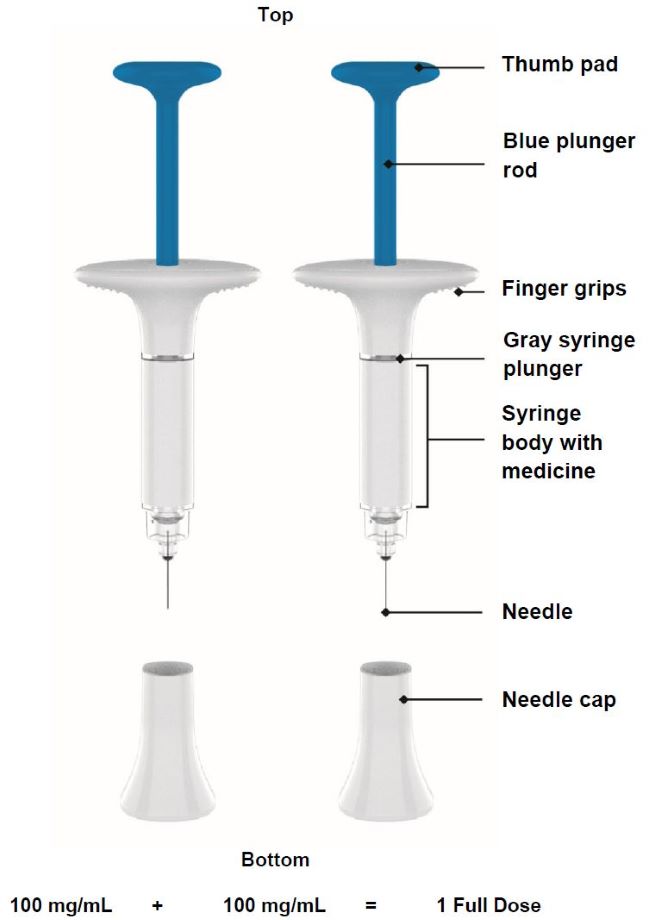 |
Important:
|
| 2 injections are required for a full dose. Inject one prefilled syringe immediately followed by the other prefilled syringe. |
| Preparing to inject OMVOH | |
| Take the prefilled syringes from the refrigerator | Take 2 OMVOH prefilled syringes from the refrigerator. |
| Leave the needle caps on until you are ready to inject. | |
| Leave the prefilled syringes at room temperature for 30 minutes before injecting. | |
| Do not microwave the prefilled syringes, or run hot water over them, or leave them in direct sunlight. | |
| Do not shake the prefilled syringes. | |
| Gather supplies | Supplies:
|
| Inspect the prefilled syringes and the medicine Expiration date 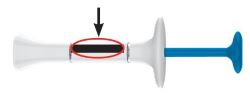 |
Make sure you have the right medicine. The medicine inside should be clear. It may be colorless to slightly yellow to slightly brown. |
Do not use the prefilled syringes and throw away (dispose of) as directed by your healthcare
provider or pharmacist if:
|
|
| Prepare for injection | Wash your hands with soap and water before you inject OMVOH. |
| Choose your injection site | Your healthcare provider can help you choose the injection site that is best for you. |
 |
|
|
|
|
|
|
|
|
|
| Clean the injection sites with an alcohol wipe. Let the injection sites dry before you inject the medicine. | |
|
|
|
|
|
|
|
|
| Read the Medication Guide for OMVOH inside this box to learn more about your medicine. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891
OMVOHTM is a trademark of Eli Lilly and Company.
Copyright © 2024, Eli Lilly and Company. All rights reserved.
Issued: April 29, 2024
OMV-0001-IFU-PFS-20240429
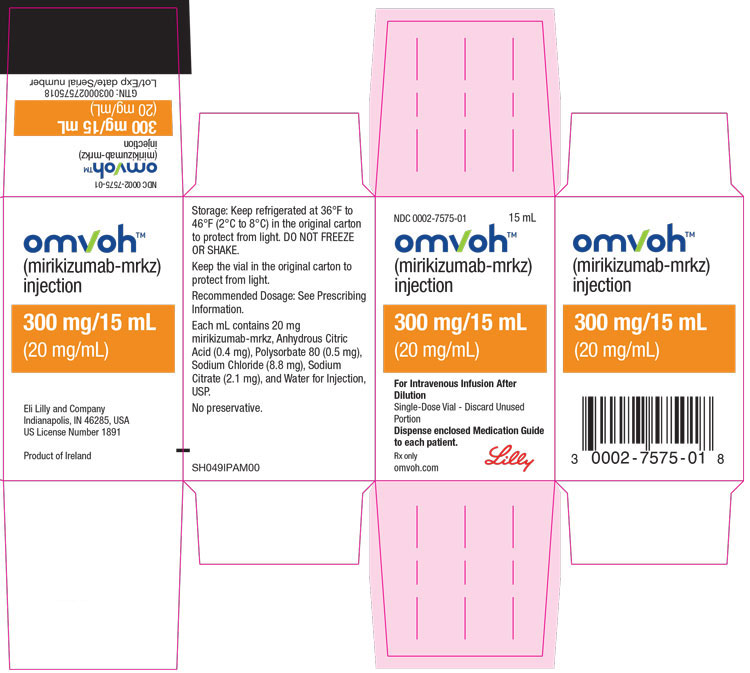
PACKAGE LABEL – Omvoh 300 mg Vial Carton
NDC 0002-7575-01
15 mL
omvohTM
(mirikizumab-mrkz)
injection
300 mg/15 mL
(20 mg/mL)
For Intravenous Infusion After Dilution
Single-Dose Vial - Discard Unused Portion
Dispense enclosed Medication Guide to each patient.
Rx only
omvoh.com
Lilly
PACKAGE LABEL – Omvoh 100 mg Prefilled Pen
NDC 0002-8011-27
omvohTM
(mirikizumab-mrkz)
injection
100 mg/mL
For Subcutaneous Use Only
For a 200 mg dose, two 100 mg pens are required. Inject one pen after the other.
2 x 1 mL single-dose prefilled pens
Rx only
Dispense enclosed Medication Guide to each patient.
Lilly
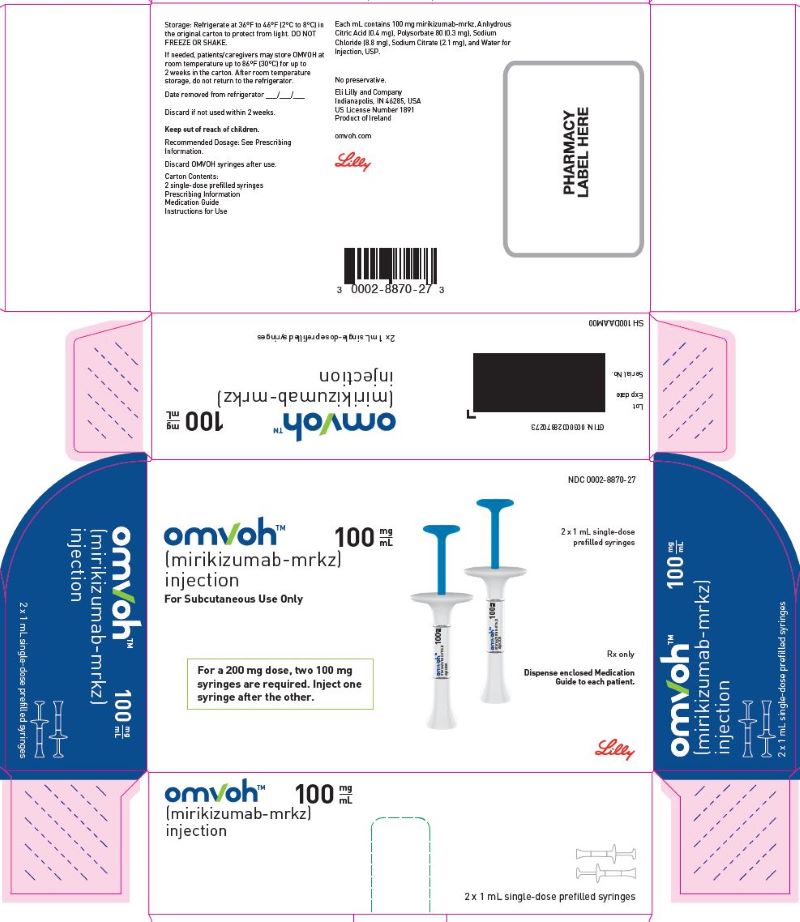
PACKAGE LABEL – Omvoh 100 mg Prefilled Syringe
NDC 0002-8870-27
omvohTM
(mirikizumab-mrkz)
injection
100 mg/mL
For Subcutaneous Use Only
For a 200 mg dose, two 100 mg syringes are required. Inject one syringe after the other.
2 x 1 mL single-dose prefilled syringes
Rx only
Dispense enclosed Medication Guide to each patient.
Lilly