FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
HUMULIN R is indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus.
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Always check the insulin label before administration to confirm the correct insulin product is being used [see Warnings and Precautions (5.4)].
- Inspect HUMULIN R visually before use. It should appear clear and colorless. Do not use HUMULIN R if particulate matter or coloration is seen.
2.2 Route of Administration
Subcutaneous Injection
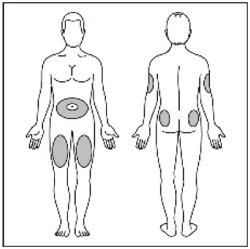
- Inject HUMULIN R subcutaneously approximately 30 minutes before meals into the thigh, upper arm, abdomen, or buttocks.
- Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- HUMULIN R may be diluted with Sterile Diluent for HUMULIN R for subcutaneous injection ONLY under medical supervision.
- Dilute one part HUMULIN R to nine parts diluent to yield a concentration one-tenth that of HUMULIN R (equivalent to U-10).
- Dilute one part HUMULIN R to one part diluent to yield a concentration one-half that of HUMULIN R (equivalent to U-50).
- Diluted HUMULIN R may be used for 28 days when stored at 41°F (5°C) or for 14 days when stored at 86°F (30°C).
Intravenous Administration
- Administer HUMULIN R intravenously ONLY under medical supervision with close monitoring of blood glucose and potassium levels to reduce the risk of hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.6)].
- For intravenous use, HUMULIN R should be used at concentrations from 0.1 unit/mL to 1 unit/mL in infusion systems containing 0.9% Sodium Chloride Injection.
- Intravenous infusion bags prepared with HUMULIN R are stable when stored in a refrigerator (36° to 46°F [2° to 8°C]) for 48 hours and then may be used at room temperature for up to an additional 48 hours.
2.3 Dosing Instructions
- Individualize and adjust the dosage of HUMULIN R based on the route of administration, the patient's metabolic needs, blood glucose monitoring results, and glycemic control goal.
- HUMULIN R given by subcutaneous injection should generally be used in regimens with an intermediate- or long-acting insulin.
- During changes to a patient's insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
- Dosage adjustments may be needed with changes in physical activity, changes in meal patterns (e.g., macronutrient content or timing of food intake), changes in renal or hepatic function, or during acute illness [see Warnings and Precautions (5.2, 5.3), Use in Specific Populations (8.6, 8.7)].
- Dosage adjustment may be needed when changing from another insulin to HUMULIN R [see Warnings and Precautions (5.2)].
2.4 Dosage Adjustment due to Drug Interactions
- Dosage adjustment may be needed when HUMULIN R is co-administered with certain drugs [see Drug Interactions (7)].
3 DOSAGE FORMS AND STRENGTHS
Injection: 100 units/mL (U-100), clear and colorless solution available as:
- 10 mL multiple-dose vial
- 3 mL multiple-dose vial
5 WARNINGS AND PRECAUTIONS
5.1 Never Share Needles or Syringes between Patients
Patients using HUMULIN R vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6)].
Make any changes to a patient's insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes, adjustments in concomitant anti-diabetic medications may be needed.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction of all insulins, including HUMULIN R. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of HUMULIN R may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature [see Clinical Pharmacology (12.2)].
Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to concomitant drugs [see Drug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between HUMULIN R and other insulins, instruct patients to always check the insulin label before each injection.
5.5 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with HUMULIN R [see Adverse Reactions (6)]. If hypersensitivity reactions occur, discontinue HUMULIN R; treat per standard of care and monitor until symptoms and signs resolve. HUMULIN R is contraindicated in patients who have had a hypersensitivity reaction to HUMULIN R or its excipients.
5.6 Hypokalemia
All insulins, including HUMULIN R, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including HUMULIN R, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling:
- Hypoglycemia [see Warnings and Precautions (5.3)]
- Hypoglycemia Due to Medication Errors [see Warnings and Precautions (5.4)]
- Hypersensitivity [see Warnings and Precautions (5.5)]
- Hypokalemia [see Warnings and Precautions (5.6)]
Adverse Reactions from Clinical Studies or Postmarketing Reports
The following additional adverse reactions have been identified during clinical studies or from postmarketing reports with use of HUMULIN R. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure.
Adverse reactions associated with insulin initiation and glucose control intensification
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. Over the long-term, improved glycemic control decreases the risk of diabetic retinopathy and neuropathy.
Hypersensitivity Reactions
Severe allergic reactions may include anaphylaxis, generalized skin reactions, rash, angioedema, bronchospasm, hypotension, and shock.
Hypokalemia
HUMULIN R can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia.
Injection Site Reactions
Injection site reactions may include injection site hematoma, pain, hemorrhage, erythema, nodules, swelling, discoloration, pruritus, warmth, and injection site mass.
Lipodystrophy
Administration of insulin subcutaneously, including HUMULIN R, has resulted in lipoatrophy (depression in the skin) or lipohypertrophy (enlargement or thickening of tissue) in some patients [see Dosage and Administration (2.2)].
Localized Cutaneous Amyloidosis
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
Medication Errors
Medication errors in which other insulins have been accidentally substituted for HUMULIN R have been identified during postapproval use.
Peripheral Edema
Insulins, including HUMULIN R, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
7 DRUG INTERACTIONS
| Drugs that May Increase the Risk of Hypoglycemia | |
| Drugs: | Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics |
| Intervention: | Dose adjustment and increased frequency of glucose monitoring may be required when HUMULIN R is co-administered with these drugs. |
| Drugs that May Decrease the Blood Glucose Lowering Effect of HUMULIN R | |
| Drugs: | Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
| Intervention: | Dose adjustment and increased frequency of glucose monitoring may be required when HUMULIN R is co-administered with these drugs. |
| Drugs that May Increase or Decrease the Blood Glucose Lowering Effect of HUMULIN R | |
| Drugs: | Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
| Intervention: | Dose adjustment and increased frequency of glucose monitoring may be required when HUMULIN R is co-administered with these drugs. |
| Drugs that May Blunt Signs and Symptoms of Hypoglycemia | |
| Drugs: | Beta-blockers, clonidine, guanethidine, and reserpine |
| Intervention: | Increased frequency of glucose monitoring may be required when HUMULIN R is co-administered with these drugs. |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published studies over decades have not established an association with human insulin use during pregnancy and major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations). Animal reproduction studies were not performed.
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7% and has been reported to be as high as 20-25% in women with a HbA1c >10%. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia-related morbidity.
Data
Human Data
While available studies cannot definitively establish the absence of risk, published data from retrospective studies, open-label, randomized, parallel studies and meta-analyses over decades have not established an association with human insulin use during pregnancy and major birth defects, miscarriage, or adverse maternal or fetal outcomes. All available studies have methodological limitations, including lack of blinding, unclear methods or randomization, and small sample size.
8.2 Lactation
Risk Summary
Available data from published literature suggests that exogenous human insulin products, including HUMULIN R, are transferred into human milk. There are no adverse reactions reported in breastfed infants in the literature. There are no data on the effects of exogenous human insulin products, including HUMULIN R on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for HUMULIN R and any potential adverse effects on the breastfed child from HUMULIN R or from the underlying maternal condition.
8.4 Pediatric Use
HUMULIN R is indicated to improve glycemic control in pediatric patients with diabetes mellitus.
The dosage of HUMULIN R must be individualized in pediatric patients based on metabolic needs and frequent monitoring of blood glucose to reduce the risk of hypoglycemia [see Dosage and Administration (2.3), Warnings and Precautions (5.3)].
8.5 Geriatric Use
The effect of age on the pharmacokinetics and pharmacodynamics of HUMULIN R has not been studied. Elderly patients using HUMULIN R may be at increased risk of hypoglycemia due to co-morbid disease [see Warnings and Precautions (5.3)].
8.6 Renal Impairment
The effect of renal impairment on the pharmacokinetics and pharmacodynamics of HUMULIN R has not been studied. Patients with renal impairment are at increased risk of hypoglycemia and may require more frequent HUMULIN R dose adjustment and more frequent blood glucose monitoring [see Warnings and Precautions (5.3)].
8.7 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of HUMULIN R has not been studied. Patients with hepatic impairment are at increased risk of hypoglycemia and may require more frequent HUMULIN R dose adjustment and more frequent blood glucose monitoring [see Warnings and Precautions (5.3)].
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise may be needed. More severe episodes with coma, seizure, or neurologic impairment may be treated with a glucagon product for emergency use or intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately [see Warnings and Precautions (5.3, 5.6)].
11 DESCRIPTION
Insulin human is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli and has the empirical formula C257H383N65O77S6 with a molecular weight of 5.808 kDa.
HUMULIN R (insulin human) injection is a short-acting human insulin for subcutaneous or intravenous use.
HUMULIN R is a sterile, aqueous, clear, and colorless solution. HUMULIN R contains 100 units of insulin human in each milliliter. Each milliliter of HUMULIN R also contains glycerin (16 mg), metacresol (2.5 mg), endogenous zinc (approximately 0.015 mg/100 units,) and Water for Injection. Sodium hydroxide and hydrochloric acid may be added during manufacture to adjust the pH. The pH is 7.0 to 7.8.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary activity of insulin, including HUMULIN R, is the regulation of glucose metabolism. Insulin lowers blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis and proteolysis, and enhances protein synthesis.
12.2 Pharmacodynamics
The time course of insulin action (i.e., glucose lowering) may vary considerably in different individuals or within the same individual. With subcutaneous use, the pharmacologic effect of HUMULIN R begins approximately 30 minutes (range: 10 to 75 minutes) after administration of doses in the 0.05 to 0.4 units/kg range (Figure 1). The effect is maximal at approximately 3 hours (range: 20 minutes to 7 hours) and terminates after approximately 8 hours (range: 3 to 14 hours). In a study that administered 50 and 100 units doses subcutaneously to obese subjects, mean time of termination of effect was prolonged to approximately 18 hours (range approximately 12-24 hours).
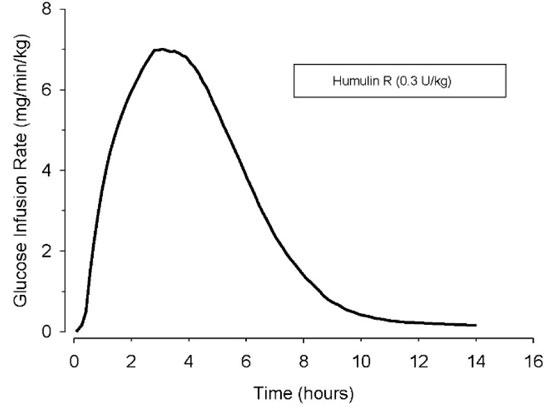
Figure 1: Mean Insulin Activity Versus Time Profile After Subcutaneous Injection of HUMULIN R (0.3 unit/kg) in Healthy Subjects.
With intravenous use, the pharmacologic effect of HUMULIN R begins at approximately 10 to 15 minutes and terminates at a median time of approximately 4 hours (range: 2 to 6 hours) after administration of doses in the range of 0.1 to 0.2 units/kg.
The intravenous administration of HUMULIN R was tested in 21 people with type 1 diabetes. During the 6-hour assessment phase patients received intravenous HUMULIN R at an initial dose of 0.5 units/hour, adjusted to maintain blood glucose concentrations near normoglycemia (100 to 160 mg/dL).
The mean blood glucose levels during the assessment phase for patients on HUMULIN R therapy are summarized below in Table 2. All patients achieved near normoglycemia during the 6-hour assessment phase. The average time (± SE) required to attain near normoglycemia was 161 ± 14 minutes for HUMULIN R.
|
a Results shown as mean ± Standard Deviation. |
|
| Time from Start of Infusion (minutes) | Mean Blood Glucose (mg/dL) Intravenousa |
| 0 | 220 ± 11 |
| 30 | 204 ± 17 |
| 60 | 193 ± 18 |
| 120 | 172 ± 28 |
| 180 | 153 ± 30 |
| 240 | 139 ± 24 |
| 300 | 131 ± 22 |
| 360 | 128 ± 18 |
12.3 Pharmacokinetics
Absorption — In healthy subjects given subcutaneous doses of HUMULIN R ranging from 0.05 to 0.4 unit/kg, mean peak serum levels occurred between 36 to 150 minutes after dosing. After subcutaneous administration of a single 12 unit dose (approximately 0.15 units/kg) to patients with type 1 diabetes, the median peak serum level occurred at approximately 2 hours (range: 20 minutes-6 hours). In a study that administered 50 units (0.4-0.6 unit/kg) and 100 units (0.8-1.3 unit/kg) doses subcutaneously to healthy obese subjects, median peak serum levels were prolonged to approximately 3 hours (range 1 to 8 hours). The absolute bioavailability after a single subcutaneous injection of HUMULIN R ranges from 48% to 89% in the 0.1 to 0.3 unit/kg dose range.
Distribution — When HUMULIN R is administered intravenously at doses of 0.1 or 0.2 unit/kg, the mean volume of distribution ranges between 0.32 to 0.67 L/kg.
Metabolism — The uptake and degradation of insulin occurs predominantly in liver, kidney, muscle, and adipocytes, with the liver being the major organ involved in the clearance of insulin.
Excretion — After subcutaneous administration of HUMULIN R doses in the 0.05-0.4 unit/kg dose range, the median apparent half-life is approximately 1.5 hours (range: 40 minutes-7 hours). Mean apparent half-life of HUMULIN R after subcutaneous administration of higher doses (50 and 100 units) to healthy obese subjects was approximately 3.6 hours (range= 1.6-8.6 hours).
When administered intravenously, HUMULIN R had a mean half-life of approximately 20 minutes at a 0.1 unit/kg dose and 1 hour at a 0.2 unit/kg dose.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
HUMULIN R injection is 100 units/mL (U-100), a clear and colorless solution available as:
| 10 mL multiple-dose vial | NDC 0002-8215-01 |
| 3 mL multiple-dose vial | NDC 0002-8215-17 |
Patients using HUMULIN R vials must never share needles or syringes with another person.
16.2 Storage and Handling
Protect from heat and light. Do not freeze. Do not use HUMULIN R after the expiration date printed on the label or if it has been frozen. See Table 3 below for storage conditions.
|
a When stored at room temperature, HUMULIN R can only be used for a total of 31 days including both not in-use (unopened) and in-use (opened) storage time. |
||||
| Not In-use (Unopened) |
In-use (Opened) |
|||
| Room Temperature (up to 86°F [30°C]) |
Refrigerated (36° to 46°F [2° to 8°C]) |
Room Temperature (up to 86°F [30°C]) |
Refrigerated (36° to 46°F [2° to 8°C]) |
|
| 10 mL multiple-dose vial 3 mL multiple-dose vial |
31 days | Until expiration date |
31 days | 31 days |
Storage of Diluted HUMULIN R and Intravenous Infusion Preparations with HUMULIN R
Diluted HUMULIN R for subcutaneous injection may be stored for 28 days when refrigerated at 36° to 46°F [2° to 8°C] or for 14 days at room temperature up to 86°F (30°C) [see Dosage and Administration (2.2)].
Intravenous infusion bags prepared with HUMULIN R may be stored for 48 hours when refrigerated at 36° to 46°F [2° to 8°C]. The prepared intravenous infusion bags may then be stored at room temperature for up to an additional 48 hours [see Dosage and Administration (2.2)].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Never Share Needles or Syringes between Patients
Advise patients using HUMULIN R vials not to share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
Hyperglycemia or Hypoglycemia
Inform patients that hypoglycemia is the most common adverse reaction with insulin. Instruct patients on self-management procedures including glucose monitoring, proper injection technique, and management of hypoglycemia and hyperglycemia, especially at initiation of HUMULIN R therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, and skipped meals. Instruct patients on the management of hypoglycemia [see Warnings and Precautions (5.3)].
Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery.
Advise patients that changes in insulin regimen can predispose to hyperglycemia or hypoglycemia and that changes in insulin regimen should be made under close medical supervision [see Warnings and Precautions (5.2)].
Medication Errors
Instruct patients to always check the insulin label before each injection to avoid mix-ups between insulin products [see Warnings and Precautions (5.4)].
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with HUMULIN R. Inform patients on the symptoms of hypersensitivity reactions and to seek medical attention if they occur [see Warnings and Precautions (5.5)].
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Revised: June 2022 |
| Patient Information Humulin® (HU-mu-lin) R (insulin human) injection, for subcutaneous or intravenous use U-100 (100 units/mL) |
|
| Do not share your syringes with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them. What is Humulin R?
|
|
| Who should not use Humulin R? Do not use Humulin R if you:
|
|
| What should I tell my healthcare provider before using Humulin R? Before using Humulin R, tell your healthcare provider about all your medical conditions including, if you:
|
|
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements. Before you start using Humulin R, talk to your healthcare provider about low blood sugar and how to manage it. |
|
How should I use Humulin R?
|
|
| Keep Humulin R and all medicines out of reach of children. | |
Your dose of Humulin R may need to change because of:
|
|
| What should I avoid while using Humulin R? While using Humulin R do not:
|
|
| What are the possible side effects of Humulin R? Humulin R may cause serious side effects that can lead to death, including:
|
|
| Treatment with TZDs and Humulin R may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure. Get emergency medical help if you have:
|
|
The most common side effects of Humulin R include:
|
|
| These are not all of the possible side effects of Humulin R. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
| General Information about the safe and effective use of Humulin R Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Humulin R for a condition for which it was not prescribed. Do not give Humulin R to other people, even if they have the same symptoms you have. It may harm them. This Patient Information leaflet summarizes the most important information about Humulin R. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Humulin R that is written for healthcare providers. For more information go to www.humulin.com or call 1-800-LillyRx (1-800-545-5979). |
|
| What are the ingredients in Humulin R? Active Ingredient: insulin human Inactive ingredients: glycerin, metacresol, and Water for Injection as inactive ingredients. Sodium hydroxide and hydrochloric acid may be added to adjust the pH. Manufactured by: Eli Lilly and Company, Indianapolis, IN 46285, USA US License Number 1891 For more information about Humulin R go to www.humulin.com. Copyright © 2007, 2022, Eli Lilly and Company. All rights reserved. |
|
LINR100-0004-PPI-20220627
Instructions for Use
Humulin® (HU-mu-lin) R
(insulin human)
injection, for subcutaneous or intravenous use
3 mL or 10 mL multiple-dose vial (100 units/mL)
Read the Instructions for Use before you start taking HUMULIN R and each time you get a new vial. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your syringes with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Supplies needed to give your injection
- a 3 mL or 10 mL multiple-dose HUMULIN® R vial
- a U-100 insulin syringe and needle
- 2 alcohol swabs
- 1 sharps container for throwing away used needles and syringes. See “Disposing of used needles and syringes” at the end of these instructions.
| Vial | Syringe |
 |
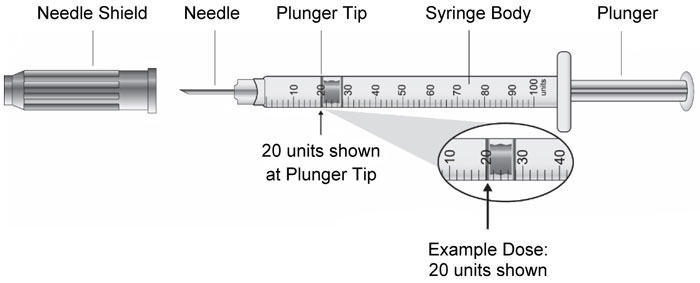 |
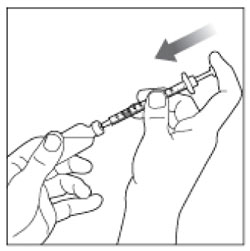
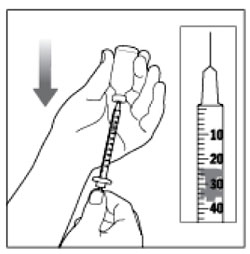
Preparing the Dose
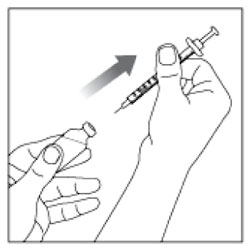
- Wash your hands with soap and water.
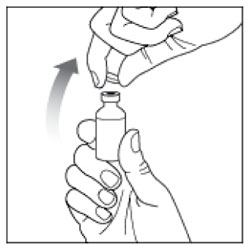
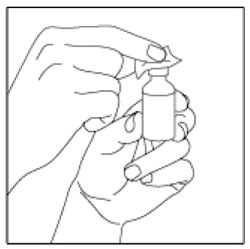
- Check the HUMULIN R label to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Do not use HUMULIN R past the expiration date printed on the label or 31 days after you first use it.
- Always use a new syringe or needle for each injection to help make sure the syringe needle is sterile and to prevent blocked needles. Do not reuse or share your syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
If you use HUMULIN R with HUMULIN N:
- HUMULIN N is the only type of insulin that can be mixed with HUMULIN R. Do not mix HUMULIN R with any other type of insulin.
- HUMULIN R should be drawn up into the syringe first, before you draw up Humulin N. Talk to your healthcare provider if you are not sure about the right way to mix HUMULIN R and HUMULIN N.
- Give your injection right away.
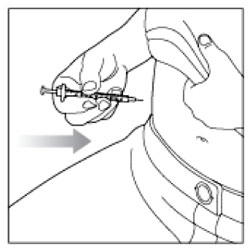
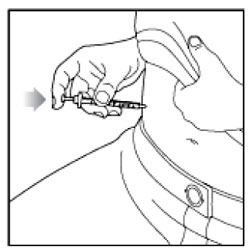
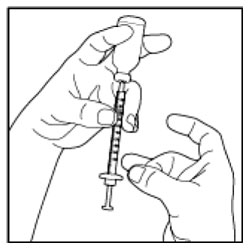
Giving your Injection
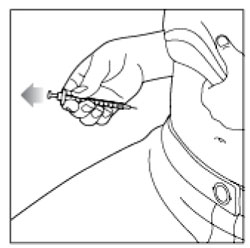
- Inject your insulin exactly as your healthcare provider has shown you.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
Disposing of used needles and syringes:
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- -
- made of a heavy-duty plastic,
- -
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- -
- upright and stable during use,
- -
- leak resistant, and
- -
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store HUMULIN R?
All unopened vials:
- Store all unopened vials in the refrigerator at 36° to 46°F (2° to 8°C).
- Do not freeze. Do not use if it has been frozen.
- Keep away from heat and out of direct light.
- Unopened vials can be used until the expiration date on the carton and label, if they have been stored in the refrigerator.
- Unopened vials should be thrown away after 31 days if they are stored at room temperature
After vials have been opened:
- Store opened vials in the refrigerator or at room temperature up to 86°F (30°C) for up to 31 days.
- Keep away from heat and out of direct light.
- Throw away all opened vials after 31 days, even if there is still insulin left in the vial.
General information about the safe and effective use
- Keep HUMULIN R vials, syringes, needles, and all medicines out of the reach of children.
- Always use a new syringe or needle for each injection.
- Do not reuse or share your syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
If you have any questions or problems with your HUMULIN, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider for help. For more information on HUMULIN and insulin, go to www.humulin.com.
Scan this code to launch the humulin.com website
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Humulin® is a trademark of Eli Lilly and Company.
Instructions for Use revised: June 2022
Manufactured by:
Eli Lilly and Company, Indianapolis, IN 46285, USA
US License Number 1891
Copyright © 1997, 2022, Eli Lilly and Company. All rights reserved.
LINR100VL-0004-IFU-20220627
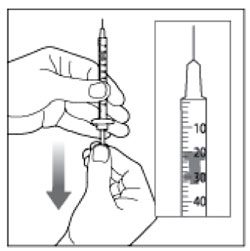 (Example Dose: 20 units shown)
(Example Dose: 20 units shown)
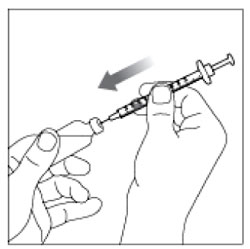
 (Example Dose: 20 units Plunger is shown at 24 units)
(Example Dose: 20 units Plunger is shown at 24 units)
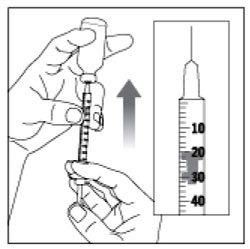 (Example Dose: 20 units shown)
(Example Dose: 20 units shown)